Acid-base lecture notes
From Biol557
- started here on 04/07/10.
- moving some lectures around
Contents
|
[edit] Acid / Base homeostasis
[edit] Acid / base 101
- There is a difference across the membrane: 7.4 blood and 7.0 in the ICF.
- Blood pH doesn't change much, only 0.1.
- The metabolic activity is what causes the low pH inside cells.
- Acid can donate hydrogen ion, base can accept.
- There are weak and strong acids and bases.
- Dissociation can be measured by ka; it is often expressed as the negative log.
- The stronger the acid the lower the negative log.
- We won't go over Kas for acids.
- We will look at pH.
- pH is the negative log of the hydrogen ion concentration.
- When using a negative log, something that is 10-14 is much lower than 10 -7.
- Anything above pH 7 is basic, below is acidic.
- Increasing blood pH over 7.8, you have death from overexcitation of nervous system, muscle tetany, convulsions.
- Lower than 6.8 will put a patient in a coma from depression of nervous system.
- So pH maintenance is important. There are several systems that do this.
[edit] Where do we get the acid equivalents?
- Mostly from metabolism.
- Breakdown of phosphoproteins generates phosphoric acid.
- Breakdown of sulfur containing amino acids generates sulfuric acid.
- Breakdown of cationic acids gives hydrochloric acids.
- Anaerobic meta = lactic acid
- Fat metabolism generates ketone bodies.
- These are just a handful of the processes that generate the acids.
[edit] Types of acids
- There are three generalized types; the different regulatory systems can handle one or more types.
- Types:
- CO2
- Fixed acids, cannot be removed from the body thorugh respiratory processes so kidney must excrete them.
- Organic acids, respiration can compensate for but not get rid of these. These are the acids produced by metabolism.
[edit] Compensation for acid production
- How do we deal with wild swings in acid and base levels?
- First is the chemical buffers inside cells and the blood stream.
- There is a bunch of buffering capacity.
- Happens immediately.
- Respiratory center
- Brain stem has center that can change breathing rate which determines how much CO2 there is in the blood and can thus compensate for alkalosis and acidosis..
- This takes minutes.
- Renal compensation
- This can take hours.
- This is the getting rid of acids and bases through excretion via the urine.
[edit] Immediate buffering systems
- How good are they?
- If you put 1 ml of 10mM of HCl into 10 ml of water, the pH goes from 7 to 3.
- If you put 1 ml of 10mM of HCl into 10 ml of blood, the pH goes from 7.4 to 7.
- So, you're immediate response is fairly phenomenal.
[edit] Buffer systems
- There are several common pairs of acids and buffers.
- All three systems (chemical, respiratory centers, kidneys) will be involved.
- Chemicals that serve as buffers break down into:
- proteins, phosphate buffers, bicarbonate / CO2.
- The respiratory system can only deal with bicarbonate / CO2.
- The kidneys can work through:
- bicarbonate / CO2
- titratable acids (these are fixed acids like sulfate, etc).
- We call them titratable because they used to be titrated to measure their levels in the urine.
- Ammonia, ammonium.
[edit] Chemical buffers
- The effectiveness is dependent on:
- strength of dissociation of acid / base (that is, the buffer)
- concentration of buffer
[edit] Proteins
- These are good because all the side groups can act as buffering units.
- They are also good because they are found in high concentration on the inside and outside of cells.
- Hb has 37 histidine residues which are good at absorbing hydrogen ions.
- Albumins are also good at buffering.
- Proteins can act as a base in an acidic environment and as an acid in basic conditions.
[edit] Phosphate buffering
- Phosphates have a pKA such that they are good at buffering at the pH of blood.
- However, they are not high enough in concentration to control buffering all alone.
[edit] Carbonic acid / bicarbonate / CO2 system
- This is important in chemical, respiratory, and renal buffering systems.
- Present in large quantities.
- Carbonic anhydrase must be present in order for this system to work.
- Recall that CA converts CO2 to H2CO3 and vice versa.
- The movement from H2CO3 to HCO3 does not require an enzyme.
- NaHCO3 can be used to store the HCO3.
- This is called the bicarbonate reserve.
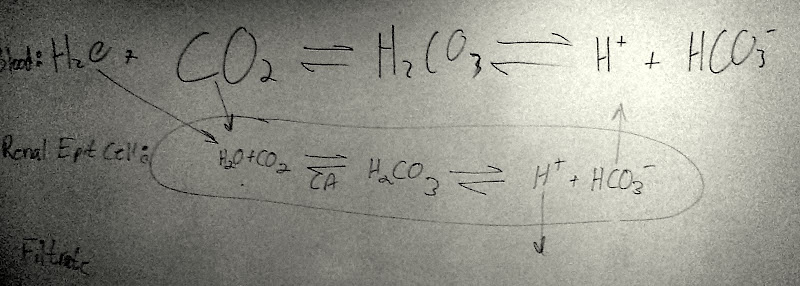
[edit] Respiratory buffering
- CO2 is exhaled at the same rate CO2 is being produced, so there is net balance.
- If you hypoventalate the CO2 is not given off so you become acidic because it pushes the reaction toward generation of HCO3.
- If you are getting rid of CO2 faster than it is being produced, it will drive the reaction to the left toward CO2 and thus the blood becomes alkaline.
- If the blood becomes acidic, then the peripheral receptors in the corotid and aortic vessels will sense the pH and signal the respiratory center that is in the brain to increase ventilation. That will decrease CO2 and increase the pH by forcing the reaction to the left.
- If the blood becomes basic, different peripheral receptors will sense this and decrease breathing and therefore retain the CO2 and shift the reaction to the right to retain HCO3- and thus decrease the pH to make the blood more acidic..
- This sensing and changing of breathing based on blood pH will work for a short time but will then be overridden by CO2 sensors in the brain.
- And this makes sense because if you stop breathing you'll die. It is more important that you keep breathing and hope that pH gets figured out through other mechanisms.
[edit] Renal buffering
- The respiratory system can only regulate the CO2. So it can compensate for fixed acids and bases in the blood but cannot change their levels.
- So the renal system must do this.
- The renal system is going to work with the CO2 / bicarbonate system and with fixed acids.
- The renal system uses CO2 / bicarb much differently than does the respiratory system.
[edit] Bicarbonate
- Renal buffering system can secrete H+ into the filtrate, reabsorb bicarbonate from the filtrate, and produce bicarbonate.
- Note that this "reabsorbing" of the bicarbonate is not direct.
- The bicarbonate that is filtered is 4500 mEquivalents / day but cannot be reabsorbed because there is not an apical channel on the ductal epithelium.
- But there is a mechanism for preserving bicarbonate.
- How is it done?
- We have bicarbonate and carbonic acid in the filtrate.
- No transporter on epithelial surface for either carbonic acid or bicarbonate.
- We do have a membrane bound CA on the apical membrane.
- Then carbonic acid can be broken down into CO2 and water via carbonic anhydrase.
- CO2 moves into cell by diffusion.
- Then an enzyme (CA) will use the H20 and CO2 and make it into H2CO3 which can then freely become HC03.
- Then an HCO3 transporter on the basal membrane moves it into the blood.
- The extra H ion goes back into the filtrate.
- Why do all this?
- It is a mechanism for controlling acid / base.
- If we just reabsorbed all the bicarbonate we would have no modulation.
- The hydrogen that is generated through this process is important for keeping the cycle going and for use with fixed acids and bases.
- If we didn't have this system only about 1% of the acids of the blood could be secreted.
- So this is how "reabsorption" of bicarbonate works in the renal buffering system.
- This whole process can be reversed, too:
- Pretend the blood is basic.
- intracellular CA genrates bicarbonate and H+.
- H+ goes into blood.
- HCO3 can't get into filtrate except by a cotransporter that is one way out (note that this is on the apical membrane).
[edit] Ammonia
- If necessary, if you need to basify the blood, we can actually make ammonia in the proximal tubule cells.
- Glutamine is broken down to form the ammonia.
- This mechanism is not normally active.
- This is up-regulated in severe acidosis, but it takes about a day.
- How does this work?
- Glutamine is broken down into NH4 and HCO3.
- HCO3 diffuses into the blood to decrease acidosis, that is, basify the blood.
- NH4 or NH3 (depending on pH of cell) will be secreted to the urine.
- This gets rid of acid and puts a base in the blood.
[edit] Titratable acids / bases
- Note that fixed acids are filtered out directly by the normal filtering process of the kidney. That is to say that fixed acids will not be reabsorbed from filtrate into the blood.
- Under acidic conditions, nephron epithelial cells compensate for the blood's low pH in the same manner that the lungs do; they generate H+ and HCO3 through carbonic anhydrase and put the HCO3 into the blood to increase the pH.
- Under basic conditions, the nephronic epithelial cells again generate H+ and HCO3 but secrete the bicarbonate.
- Remember that CO2 is a gas that can diffuse readily.
[edit] Disturbances of acid / base balance
- On the exam, if we say acidosis or alkalosis we must clarify whether it is a respiratory or metabolic acidosis / alkalosis.
- If the problem is with fixed acids or bases it is a metabolic disturbance.
- If the probelm is with CO2 it is a respiratory disturbance.
[edit] Respiratory acidosis
- Generally caused by a CO2 issue.:
- Biggest cause is pulmonary disease where you cannot get rid of CO2 because you cannot exhale. This is high compliance.
- There could be insufficient neural drive, that is, a breathing rate that is too slow.
- Common causes:
- Pneumonia, CF (scarring decreases gas exchange), emphasema, pulmonary adema.
- All inhibit gas exchange.
- Narcotic or barbituate overdose.
- Brain stem injury.
- How can we treat it?
- Depends on what causes the problem.
- We can do artificial respiration if nerual or muscular issue.
- IV bicarbonate can be administered.
- How does the body deal with this?
- Chemical buffering, we'll ignore this because it is always there.
- Respiratory
- Peripheral sensors will notice decrease in pH and increase breathing rate.
- In pulmonary edema (where the lungs or breathing are the problem in the first place) this won't help much.
- Peripheral sensors will notice decrease in pH and increase breathing rate.
- Renal reaction
- As we've seen before, CO2 and H20 will be taken from the blood to be generated into H+ and HCO3. ***Then H+ ions are secreted and HCO3 is pumped into the blood.
- This takes up to a day to kick.
[edit] Respiratory alkalosis
- Cuased by hyperventilation:
- Voluntary, could be crying for long periods of time
- Anxiety
- Stimulation of respiratory centers
- Aspirin toxicity (don't take lots together, often)
- High fevers can affect resp center.
- Meningitis
- Reflex action at high altitudes
- Because partial pressure of O is low, we start taking more and deeper breaths. This causes hyperventillation and causes CO2 in the blood to go down.
- So all-in-all this is a net loss of CO2.
- What does the body do about it?
- Chemical system
- Respiratory
- Reduces breathing rate
- Renal
- Kidneys secrete bicarbonate (hydrogen goes back into the blood).
- Treatment:
- Breath in a paper bag which will increase partial pressure of CO2 and decrease release of CO2.
[edit] Metabolic acidosis
- Causes:
- Renal failure
- If you can't get rid of fixed bases then you will end up acidotic.
- Uncontrolled diabetes; ketone body formation causes acidosis.
- Excess exercise
- Generates transient acidosis state because of lactic acid.
- Don't need to worry about this
- Ingestion, think ammonium chloride
- Loss of bicarbonate by diarrhea
- We often put bicarbonate into the GI to get rid of acids so if we have diarrhea, it all goes out.
- Excess alcohol consumption
- Metabolites are acidic (think acetic acid).
- Renal failure
- What does our body do?
- Chemical buffers.
- Respiratory centers
- Peripheral notice low pH and increases ventilation to decrease CO2.
- Renal
- Reclaims all the bicarbonate; turns it into CO2 in the lumen, puts it in the cell, turns it back into bicarbonate, puts it in the blood.
- If it goes on for a long time, kidney will start making ammonia out of glutamine to put even more (that is, new) bicarbonate into the blood.
- Treatment:
- IV bicarbonate.
[edit] Metabolic alkalosis
- Caused by:
- Ingestion of excess base; perhaps from overuse of antacids, don't use baking soda because it is sodium bicarbonate!
- Better to use antacids so they are not so readily absorbed.
- Vomiting
- This happens because when you vomit you get rid of stomach acids to the stomach starts making more acids. It does this by secreting an H+ ion and putting HCO3 into the blood stream.
- Some diuretics
- Cause potassium depletion because they secondarily cause H+ secretion.
- Ingestion of excess base; perhaps from overuse of antacids, don't use baking soda because it is sodium bicarbonate!
- What does the body do?
- Chemical buffers
- Respiratory system
- Sensors see high pH and send the signal to hypoventilate. This will only last until CO2 builds up to trigger the brain to say "hey, start breathing, moron".
- Renal
- Stops "reabsorbing" bicarbonate.
- Increases bicarbonate secretion.
- Treatment:
- Administer ammonium chloride. In the liver, ammonium chloride will be broken down such that H+ ions are generated.
- end of acid / base.
- test on Monday
- We'll start with PKD then GI.
- stopped here on 04/07/10.
