P's exam 3 study guide
From Iusmhistology
Revision as of 20:02, 13 April 2011 by 149.166.24.214 (Talk)
- started here on 03/21/11.
Urinary 1
- The kidney has one of the most complex 3 dimensional organizations of all the organs of the body.
- We will study the kidney incrementally, beginning with the uriniferous tubule.
- We will study some simpler kidneys that contain only one papillary, called "unipapillary kidneys".
- The functional unit of the kidney is the uriniferous tubule.
- We have 1 million uriniferous tubules.

Cortex and Medulla
- The kidney has two major regions: the cortex on the outside and the medulla on the inside.
- The pelvis is the sinus area of the kidney that is "sub-medulla" and forms the collecting area for urine before it enters the ureter.
- Urine is produced by lobes which contain a single renal papillum which dumps urine into the pelvis which dumps into the ureter.
- Small mammals often have only one lobe and therefore one renal papillum.
- Humans have multiple lobes and therefore multiple renal papilla.
- In unipapillary kidneys, the uriniferous tubules run all the way from the cortex to the papillum-pelvic border.
- The urine drips off the papilla (papillum) into the pelvis.


More on macrostructure
- Each uriniferous tubule is situated in one of the many medullary rays and medullary pyramids found in humans.
- The cortical region contains the glomeruli and is called the medullary ray.
- The medullary area contains the vasa recta, the loop of Henle, and the collecting duct.
- Medullary pyramids are separated by renal columns of Bertin.
- The renal pelvis is the area where the ureter begins to form from the sinus of the kidney.

Uniferous tubule function
- The uriniferous tubule is made up of epithelial cells.
- The tubule is surrounded by two sets of capillaries:
- The glomerular capillaries are within the Bowman's capsule in the cortex.
- The peritubular capillaries are within the medulla, along the length of the loop of Henle and the collecting duct.
- The renal corpuscle is the glomerulus, Bowman's capsule, and the glomerular capillaries.
- The renal corpuscle's function is to filter the plasma passing through the glomerular capillaries.
- The nephron and collecting describes everything other than the renal corpuscle.
- The nephron and collecting duct serve to secrete waste products and reabsorb nutrients to / from the filtrate.
- Note that the kidney filters by throwing out everything and then collecting back the things it wants to keep.
- This is good because the kidney doesn't have to know what needs to be gotten rid of which could be infinite things; the kidney only needs to know what it wants to keep.

Uriniferous tubule layout and embryonic development
- Though we draw the uriniferous tubule as a simple, linear tract, it is rarely this simple in final 3D form.
- It is important to understand embryonic development to understand why the uriniferous tubule takes its certain and functional 3D form.
- For proper functioning, it is critical that certain sections of the uriniferous tubule lie next to one another.
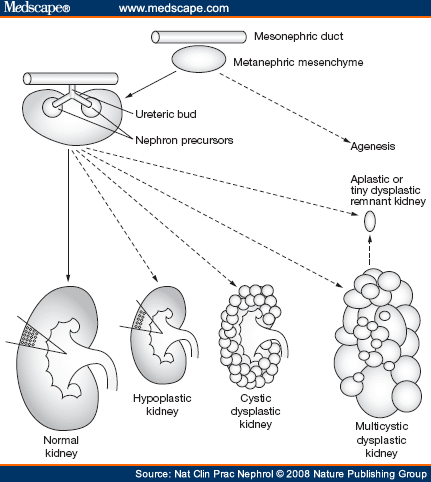
- The first form of a plasma filtering mechanism in the developing human embryo is called the mesonephric kidneys.
- Mesonephric kidneys reach their maximum size at 8 weeks and then undergo a large change.
- Parts of the mesonephric kidneys persist in men to form:
- the efferent ductules,
- the epididymis,
- the ductus deferens, and
- the ejaculatory duct.
- The cloaca is an early developing orifice that serves to excrete feces and urine.
- The cloaca is common between placental mammals, birds, amphibians, etc.
- The cloaca is retained by birds, amphibians, and reptiles.
- The cloaca in mammals divides and conributes to the anus and the urethra / vagina.
- The early plasma filtering structure is divided into two sections: the mesonephros and the metanephros.
- The metanephros gives rise to the permanent kidneys.
- The metanephros contains the metanephric mesenchyme and the uritic bud.
- The uritic bud and the metanephric mesenchyme are both composed of epithelial cells.
- The uritic bud grows up into the nephrogenic mesoderm which is part of the metanephros.
Uritic bud and nephrogenic mesoderm interaction
- The uritic bud grows into the nephrogenic mesoderm to form the mature uriniferous tubules.
- The interaction between the uritic bud and the nephrogenic mesoderm is called reciprocal induction.
- Reciprocal induction: "... tissues causing changes in each other due to signals and receptors in each" per this paper
- If the bud doesn't grow up into the nephrogenic mesenchyme, neither tissue becomes what it should.
- Reciprocal induction: "... tissues causing changes in each other due to signals and receptors in each" per this paper
- As the uritic bud grows into the nephrogenic mesenchyme, the uritic bud is the primary epithelial cell tubule structure that will become the collecting duct.
- Recall that mesenchymal cells are connective tissue cells.
- Recall that mesenchyme looks like loose connective tissue with lots of spindly, undifferentiated cells within.
- Renal corpuscles develop along the length of the uritic bud (that is, the developing collecting duct) and therefore can originate from the tip of the uritic bud or from epithelium that develops along side the uritic bud.
- Renal corpuscle and nephron development from the tip of the uritic bud:
- At the tops of the uritic bud, mesenchymal cells of the nephrogenic mesenchyme condense and are induced to make a mesenchymal-epithelial transition (MET).
- Condensation includes proliferation
- These MET cells will become the epithelial cells of the glomerular capsule.
- The bud tip then expands to develop the PCT (proximal convoluted tuble), loop of Henle (LoH), and the DCT (distal convoluted tubule).
- The MET shifted cells of the early glomeruli recruit the formation of blood vessels that will become the glomerular capillaries.
- At the tops of the uritic bud, mesenchymal cells of the nephrogenic mesenchyme condense and are induced to make a mesenchymal-epithelial transition (MET).
- Renal corpuscle and nephron development adjacent to the uritic bud
- Along side the uritic bud, epithelial tracts form as S-shaped or comma-shaped tubule structures.
- The tops of these se epithelial tracts will become the glomeruli and the length will become the PCT, LoH, and the DCT.
- The s-shaped buds from condensation, proliferation, and MET of mesenchymal cells will form the PCT, LoH, and DCT.


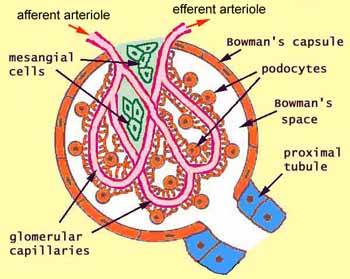
Renal corpuscle structure
- The renal corpuscle demonstrates the unique development of the uriniferous tubule by the way the podocytes surround the glomerular capillaries.
- Note that podocytes are a type of epithelial cell.
- Capillaries are a type of endothelial cell.
- We call the glomerulus the glomerular tuft before fully developed.
- Within the capillaries as they develop within the glomerular tuft, there is connective tissue holding the capillaries in place.
- This connective tissue is called mesangium.
- Bowman's space is the epithelial tract that surrounds the tuft of capillaries.
- Bowmans capsule is made of simple squamous epithelium.
- Note that this place forms a complex structure surrounding the many, convoluted, cross-connected capillaries within.
- There are many cell types and structures of the renal corpuscle; each cell type has a specific location and function.
- The afferent arteriole is made of endothelial cells and brings blood to the glomerular capillaries.
- The efferent arteriole is made of endothelial cells and takes blood away from the glomerular capillaries (to the peritubular capillaries).
- The Bowman's capsule is made of epithelial cells and surrounds the glomerular capillaries, forming the Bowman's space beween the Bowman's capsule and the walls of the glomerular capillaries.
- The inside layer of the Bowmans capsule covers the convoluted capillaries and is called the visceral layer; the parietal layer is the outside layer that forms the outer barrier of the glomerulus and is continuous with the epithelium of the PCT.
- The visceral bowmans capsule is made up of podocytes.
- The Bowman's space is the location into which filtrate is first formed by being pressed out of the plasma by hydrostatic forces (primarily, but also including colloid osmotic pressures).
- Often there is pink material in the bowmans space; it is brush border from the proximal tubule that has washed backward during fixation.
- The epithelial cells of the Bowman's capsule are continuous with the epithelial cells that make up the proximal convoluted tubule which carries filtrate.
- The distal convoluted tubule (which is, like the PCT, made up of epithelial cells) passes by the afferent arteriole along side the glomerulus.
- The DCT has specialized cells called 'macula densa cells on the surface that is closest to the afferent arteriole.
- Macula densa cells release signals PGE2 to cause the afferent arteriole to vasodilate and ATP to cause the afferent arteriole to constrict.
- Macula densa cells are more columnar, stain darker, and have rounder nuclei than the endothelail cells of the DCT.
- Juxtaglomerular cells (also called granular cells) are endothelial cells of the afferent arteriole that contain granules of renin.
- Granular cells (AKA juxtaglomerular cells) have a large, flattened nucleus, that is more prominent than the nucleus of lacis (extraglomerular mesangial) cells.
- Granular cells release their renin upon PGE2 binding their EP4 receptor.
- Recall that renin will activate angiotensinogen leading to angiotensin 2 and systemic vasodilation.
- Lacis cells (also called extraglomerular mesangial cells) hold the DCT, the afferent arteriole, and the glomerulus together.
- Extraglomerular mesangial cells may also have some functioning in modifying the signals released by the macula densa cells as they travel to the granular / endothelial cells of the afferent arteriole.
- Lacis cells (extraglomerular mesangial cells) are found between the macula densa cells and the afferent arteriole endothelial cells.
- Lacis cells have a lighter stain and less prominent nucleus as compared to granular (juxtaglomerular) cells.
- This makes sense because granular cells will have granules full of the protein renin.
- Extraglomerular mesangial cells are found between the convoluted capillaries, too, and serve to hold the loops in their structure.
- In this case, the mesangial cells are located within the basement membrane.
- Lacis cells can send processes into the lumen of the capillaries between the endothelial cells.





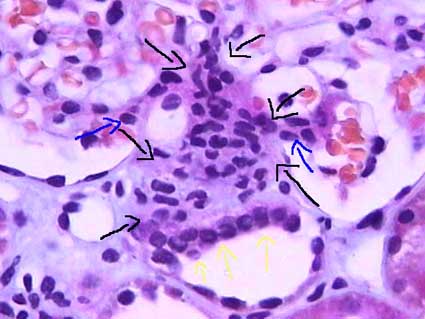
Forming a filter at the capillary-Bowman-space junction
- There are three levels of filtration at the capillary-Bowman-space junction.
- The filtrate must first get through the endothelium of the capillary, then through the basement membrane, and then through the feet of podocytes.
- The endothelium of glomerular capillaries is fenestrated without diaphragms to allow only very small proteins and smaller molecules through.
- The basal lamina does restricts even the smallest proteins.
- There are three layers to the basal lamina (basement membrane) of the glomerulus.
- The three layers are probably only separate in slides as a result of processing, but they are still effective markers for pathology.
- The lamina rara extrna is farthest from the lumen of the capillary.
- The lamina rara interna is closest to the lumen of the capillary.
- The lamina densa is between the lamina externa and the lamina interna.
- These layers appear as a light-dark-light pattern in EM.
- Podocytes are a type of epithelial cell that provide the finest level of filtration of the plasma as it crosses into the Bowman space.
- Podocytes project feet that sit on the outside (that is, the Bowman space side) of the capillaries.
- Podocytes often interdigitate to provide a nice tight filter.
- Podocytes form slit pore diaphragms which are very small and let only small molecules through to the bowmans space.
- Water and small molecules pass freely into the Bowman space.
- It is still disputed what factors play the primary role in keeping proteins from entering the filtrate.
- Some say the anionic charge of the basement membrane, which would repel proteins which are generally negatively charged, is the primary factor that hinders protein passage.
- Others point to the podocyte processes and the important proteins that make up the processes (ZO1, nephrin, Neph1) as the primary protein-hindering mechanism.
- Nephrin seems to form a lattice between podocyte processes that would prevent proteins from passing into the bowman space.

- Recall that ZO1 is associated with tight junctions.
Mesangial cells
- Recall that mesangial cells reside between capillaries within the basement membrane.
- Recall that basement membranes are always made of type 4 collagen!
- Mesangial cells may modulate capillary blood flow.
- Mesangial cells may also act as phagocytes within the basement membrane of the glomerulus.
- Mesangial cells reaches out and cups each capillary around it.
- Mesangial matrix is made up of collagen, glycans, proteoglycans, etc.



The proximal tubule
- The proximal tubule's primary function is reabsoprtion.
- Approximately 2/3 of the filtrate is reabsorbed in the PT (proximal tubule).
- The proximal tubule is characterized by being large, being eosinophilic (cuboidal, continuous, uniform), and having central nuclei.
- Eosinophilic means the cells will stain very pink.
- The epithelium of the proximal tubule is a simple squamous epithelium.
- The proximal tubule demonstrates cells with brush border and basolateral membrane folding in order to increase its surface area.
- Note that during fixation, the brush border often sloughs off into the lumen.
- The proximal tubule is made up of the proximal convoluted tubule and then then proximal straight tubule' which then proceeds into the descending loop of Henle.
- The proximal straight tubule continues through the outer stripe of the outer medulla.
- "Straight segments ... terminate at a remarkably uniform level ... that establishes the boundary between the inner and outer stripes of the outer ... medulla." per wikipedia
- Note that this is true for both cortical- and juxtamedullar glomeruli-derived proximal straight tubules.
Cell distinction along the PCT, LoH, and DCT
- Recall that the cells of the PCT, LoH, and DCT are all epithelial cells specialized for reabsorption and / or secretion.
- There are four regions that can be distinguished by cell morphology and characteristic: PCT / thick descending limb, thin descending / thin ascending, thick ascending / DCT, and the collecting duct.
- Note that the thick descending tubule is the same as the proximal straight tubule; the same goes for the distal region: distal straight tubule = thick ascending tubule.
Cells of the PCT and PST
- Note that the PST = proximal straight tubule = thick descending / proximal loop.
- There are only epithelial cells in the PCT and thick descending loop.
- Epithelium of the PCT is a simple squamous epithelium.
- Recall that the PCT reabsorbs 70% of the filtrate; therefore it makes sense that the cells of the PCT and thick descending tubule are the only cells with a brush border.
- Cells of the PCT and thick descending tubule also have nuclei that are spaced far apart.
- PCT / thick descending tubule epithelial cells stain very pink.
- PCT / thick descending tubule cells are interdigitated.
Cells of the thin descending and thin ascending tubules
- There are only epithelial cells in the thin descending and ascending tubules.
- Recall that the descending loop is passively, highly permeable to water and solutes.
- Recall that the ascending loop is impermeable to water and actively secretes Na and Cl.
- The epithelial cells of the thin regions are thin cells that stain lightly.
- The nucleus of epithelial cells of the thin tubules is smaller than other nuclei of tubular epithelial cells.
Cells of the DST and DCT tubules
- Note that the DST = distal straight tubule = thick ascending / distal tubule.
- The epithelium of the DCT and thick ascending tubule is thicker than the PCT and thick descending tubule.
- There are three cell types in the thick ascending and DCT tubules: epithelial cells, macula densa cells, and principal cells.
- Recall that the thick ascending tubule and the DCT are the hormone-responsive regions with many ion transporters to reabsorb Na and Cl in exchange for K.
- The thick ascending tubule is called the "diluting segment" of the nephron because solutes are removed from the filtrate and the epithelium is not very permeable to water, thus making the filtrate more dilute as solutes are reabsorbed and water cannot follow.
- Water at the DST is pretty dilute: 60 mOsm relative to blood's 285 mOsm.
- The DCT is considered part of the LoH.
- Epithelial cells of the thick ascending tubule and DCT need lots of protein to facilitate ion transport and so it makes sense that thick ascending epithelium and DCT epithelium have lots of mitochondria.
- Epithelial cells of the thick ascending tubules and the DCT have apical nuclei that bulge outward (perhaps because of the mt that are pushing them apically).
- Recall that epithelial cells of the DCT will include macula densa cells.
- Macula densa cells appear at the last part of the thick ascending tubule.
- Macula densa cells stain darker than other epithelial cells and are more columnar.
- Macula densa cells are found at the vascular pole of the glomerulus, near the endothelial cells of the afferent arteriole.
- It makes sense that the urinepherous tubule's own DST is near its glomerulus because they started growing at the same time from the same location (recall the MET transition and uritogenic bud).
- The DCT is the first site of intercalated cells.
Differentiating PST and DST
- Thick descending and thick ascending can be differentiated by their stain and the intracellular location of their nuclei:
- Thick descending epithelium stain darker than thick ascending epithelium.
- Thick descending epithelium has more basally located nuclei while ascending epithelium have apically located nulcei.
- Thick descending has a thicker wall than the thick ascending.
PCT versus PST and DCT versus DST identification
- Note that PST and PCT can be differentiated because they are never found in the same location: PCT is in the convoluted area and PST is only in the medullary ray area.
- The DST and DCT cannot be differentiated because the DST spans the convoluted area and medullary ray area of the cortex and runs through the outer medulla. The DCT resides only in the cortex.
- Because the DCT and DST are both bound in the cortex, it is likely impossible to tell them apart (unless the structure in question runs right up next to a glomeruli and has macula densa at which point we know it is a DST).


Differentiationg PCT and DCT
- PCT and DCT can be distinguished by their stain and size:
- PCT epithelium has a brush border but DCT epithelium does not, though often the brush border is not preserved.
- PCT stains darker than DCT, though sometimes it can be the opposite, so good luck with that.
- PCT is made of larger cells than DCT (so with PCT you travel farther around the tubule before finding the next nucleus).

Cells of the collecting duct
- There are two types of epithelial cells in the collecting duct: principal cells and intercalated cells.
- Recall that the collecting duct's function is to reabsorb water--to concentrate the urine.
- Epithelial cells of the collecting duct are characterized by large, weakly staining (even clear) cells that bulge into the lumen.
- Epithelial cells of the collecting duct have clear distinctions between each cell and have nuclei that do not bulge (like PCT / thick ascending tubule epithelial cells).
- Nuclei are more basal and irregularly shaped.
- Principal cells are hormonally controlled for water reabsorption and are the major site of potassium regulation.
- Principal cells absorb Na and secrete K.
- Principal cells are generally impermeable to water but can become water absorptive when ADH is present (think AQ2).
- Intercalated cells stain darkly, bulge a little into the lumen, have no brush border, have a more apical nucleus than principal cells, and are the site of pH regulation.
- There are three sections to the collecting duct: the connecting tubule and cortical collecting tubule, the outer medullary collecting tubule, and the inner collecting tubule.
- The two proximal sections (connecting duct / cortical collecting duct and the outer medullary collecting duct) have principal and interstitial cells; the inner medullary collecting duct has only principal cells.
- The inner medullary collecting duct is also called the papillary collecting duct.
- The last section of the inner medullary collecting duct is called the duct of Bellini.
Distinguishing regions of the kidney
- Note that thin segments of the LoH and DCT / PCT never occur in the same area so they can be used to determine the origin of a section.
- Thin loops of Henle are only found in the medulla.
- Recall that the thick proximal tubule terminates at the outer-inner stripe border of the medulla.
- Convoluted tubules are only found in the medulla.
- Thin loops of Henle are only found in the medulla.
- Distinguishing the medulla:
- The inner medulla has only asc / desc thin tubules and the collecting duct.
- The inner stripe of the outer medulla has asc / desc thin tubules, proximal / distal thick tubules, and the collecting duct.
- The outer stripe of the outer medulla has only thick tubules and collecting duct.
- There are no glomeruli in the medulla!
- stopped here on 03/21/11.
- started here on 03/23/11.
Urinary 2
Some details from Urinary 1
- Recall that proximal tubule epithelial cells are eosinophilic and have central nuclei.
- Recall that the brush border is usually sloughed off.
- Recall that the proximal tubule has basolateral folds and a brush border to increase the surface area.
- Note the difference between the proximal convoluted tubule and the proximal straight tubule: the straight tubule descends through the outer medullary stripe while the proximal convoluted tubule is confined to the renal cortex.
- Technically the "glomerulus" is the group of capillaries in the capsule.
Loop of Henle
- Recall that the thin descending loop of Henle is permeable to water and solutes.
- Recall that the thin ascending loop of Henle is impermeable to water and active NaCl reabsorption occurs.
- Recall that the macula densa senses the amount of NaCl in the filtrate.
- When the NaCl level is high, we want slow the filtrate flow rate so we have time to reabsorb all that valuable NaCl; therefore, when the NaCl level in the filtrate is high macula densa cells release ATP to constrict the afferent arteriole and decrease GFR.
- Conversely, very little NaCl in the filtrate at the macula densa means that the filtrate has had lots of time to have its NaCl reabsorbed so we can speed up GFR. In this case, macula densa cells release prostaglandins that cause renin release (and subsequently vasodilation) at the afferent arteriole.
More kidney superstructure
- Filtrate is dumped into minor calyces which join to form major calicies, which form the renal pelvis, which join to form the pelvic hilum, which is continuous with the ureter.

- Note that the ascending thick tubule is deeper than the descending thick tubule.
- Arcuate vessels follow the boundary of the cortex and medulla, giving off interlobular vessels that give off afferent arterioles and receive stellate vessels.
Do cortical nephrons really not have vasa recta? True! Superficial (cortical) and mid-cortical glomeruli don't have vasa recta. Furthermore, juxtamedullar glomeruli don't have peritubular capillaries. See "Renal vasculature".
- Note that other than the blood vessels that go in and out of the glomerulus (afferent and efferent arterioles), the blood vessels are not specific to a certain nephron (urinepherous tubule); one vessel can service more than one tubule.
- The thick descending tubule = proximal straight tubule = pars recta.
- The human kidney is multilobar and each lobe has a single medulla called "pyramid".
- The cortical tissue of adjacent pyramids (medulla) converge and also, together, run deep toward the renal hilum to form the columns of Bertin.
- Note that renal corpuscles can reside in these columns of Berin but their nephron segment will still reside in one of the neighboring medullary pyramids.
- Collecting ducts empty filtrate into the calyces at the papillary duct.
- Kidney stones (calcification or sedimentation of minerals) can form in the calyces of the kidney.
- We can remove kidney stones through a surgery that pierces the cortex, enters a calyx, and uses a probe to grab / destroy the stone. Percutaneous nephroscopy.
- Kidney stones are painful.
- Stones often form right on the tip of the papilla.
Renal vasculature
- The order of renal blood flow: renal artery -> interlobar artery -> arcuate artery -> cortical radial artery (imagine these radiating outward from the arc; used to be called interlobular arteries) -> afferent arteriole -> glomerular capillaries -> efferent arteriole.
- The return route can start from two locations:
- Superficial and mid-cortical glomerulus: (from efferent arteriole) peritubular capillaries
- superficial peritubular capillaries return via stellate veins -> arcuate vein...
- deeper peritubular capillaries return via cortical radial vein -> arcuate vein...
- Juxtamedullary glomerulus: (from efferent arteriole) descending vasa recta -> ascending vasa recta -> arcuate vein...
- Superficial and mid-cortical glomerulus: (from efferent arteriole) peritubular capillaries
- Then both follow the same path away from their respective nephron: arcuate vein -> interlobar vein -> renal vein.

Cortex organization
- A renal lobule is a unit of renal tissue with medullary ray at the center with cortical radial vessels bounding it on the outsides.
- The cortex contains medullary rays, extensions of tubules from the medulla.
- "Under low power the cortex is divisible into alternating bands called the cortical labyrinth, which is recognized by the presence of numerous renal corpuscles and medullary rays, relatively straight collections of epithelial tubules oriented perpendicular to the capsule." per SUNY Downstate Medical
- So medullary rays are the ascending and descending tubules that will run perpendicular to the capsule of the kidney.
- So, a cortical labyrinth is a collection of renal corpuscles with their associated medullary rays.
- Note that in the cortex there is both DCT / PCT (which will run every which way) as well as proximal / distal straight tubules which will run down into the medulla. It is the straight tubules that form medullary rays within cortex cuts.
Juxtuloglomerular apparatus and the renin-angiotensin pathway
- Renin is released by granular cells (juxtaglomerrular cells).
- Renin cuts angiotensinogen into angiotensin 1 which is cut by angiotenins converting enzyme into angiotensin 2 (at the lungs).
- Angiotensin 2 causes systemic vasodilation.
- At the kidney, angiotensin2-caused vasodilation increases the GFR.
- Juxtaglomerular cells (granular cells) have granules full of renin; the granules can be seen in many slide preparations.
- The apparatus contains the afferent and efferent arterioles, the macula densa, and the extraglomerular cells (lacis cells).
- There are also juxtaglomerular cells which are smooth muscle / endocrine cells.
- Also called "granular cells".
- There are also juxtaglomerular cells which are smooth muscle / endocrine cells.



Post-kidney urinary ultrastructure
- After the minor, then major calyces and the renal pelvis, filtrate (urine) enters the ureter, then the urinary bladder, then the urethra.
- The calyces, pelvis, ureters, bladder, and uretra all have the same histological structure.
- The only exception is that the walls of the ureters become thicker as they continue.
- The calyces through bladder are transitional epithelium with a lamina propria and smooth muscle.
- The urethra is sometimes transitional epithelium, too, depending on whether male or female.
- Transitional epithelium allows these structures to change volume easily, which is most obviously important in the bladder.
- Note that when no distended the cells look as if they are stacked on top of one another, then they are thin and spread out when distended.
- Transitional epithelium cells are characterized by a bulging apical surface (umbrella cells) and may be bi- or poly- nucleate.
- The lamina propria holds the cells together with connective tissue when changing volume.
- The smooth muscle allows contraction for movement of urine along the tract.
Transitional epithelium of the bladder
- The transitional epithelium of the bladder has a special mechanism for expanding and contracting its surface area.
- Uroplakins can fold up like a pleat.
- A protein called uroplakin can be moved to the surface or removed from the surface via vesicular movement in order to increase or decrease surface area.
- Vesicles that contain uroplakin are called fusiform cytoplasmic vescicles.
- Uroplakin, as with all membrane proteins, is generated via the rER and golgi apparatus.
- We also know that there is some normal turnover of uroplakin; that is, there is an equilibrium of lysosomal-breakdown and rER-golgi-production.
What is "IC" and "UC" on slide 74.
- Where uroplakin is on the surface, the membrane is thicker; there are thinner areas of membrane that are distict in EM of bladder epithelium.



Smooth muscle of the bladder
- Smooth muscle of the calyces, pelvis, and ureters are helical in pattern.
- Smooth muscle in the bladder is longitudinal and runs in all directions.
Be able to draw the whole urinipherous tubule and all the regions.
- stopped here on 03/23/11.
- started here on 03/24/11.
Endocrine histology
Describe the structural organization of the endocrine system
- The endocrine has several specific characteristics:
- cells of epithelial origin secrete hormones onto endothelial tracts (that is, the blood stream)
- hormones act at distance sites defined by having the receptor for the hromone
- secretory cells are localized to endocrine organs
- hormones can be classified into one of several grooups: amino acids, peptides, steroids, or proteins / glycoproteins
- Note that exocrine glands secrete onto an epithelial surface that is usually in the form of a duct whereas endocrine glands secrete into the blood stream.
- Furthermore, both exocrine glands and endocrine glands are usually on the outside of the basement membrane relative to the blood.
- Therefore, exocrine glands do not secrete across the basement membrane.
- However, endocrine glands often must secrete their hormones across the basement membrane.
Define components of the endocrine system
- There are three types of endocrine components: full endocrine organs, endocrine components as part of other solid organs, and diffuse endocrine components.
- Full endocrine organs are those organ in which the primary function is to synthesize, store, and secrete hormones.
- Think pituitary gland.
- Endocrine components as part of a solid organ are those organs in which there are clusters of endocrine cells.
- Think pancreas.
- Diffuse endocrine components occur when individual endocrine cells are scattered (or clumped) within an extensive epithelium.
- Think adipose tissue and sabaceous glands.
Describe the embryonic origin, histological organization, and hormone secretion of the endocrine system
Origin, organization, and secretion of the hypothalamus
- The hypothalamus resides in the lower, central part of the brain and houses neurosecretory neurons that produce hormones.
- Neurosecretory neurons secrete hormones into the hypothalamic-hypophysis portal system, a double capillary bed that facilitates potent delivery of hormones from the hypothalamus to the hypophysis (pituitary).
- The hypothalamus releases 5 hormones from three nuclei (dorsal medial, ventral medial, and infundibular nuclei):
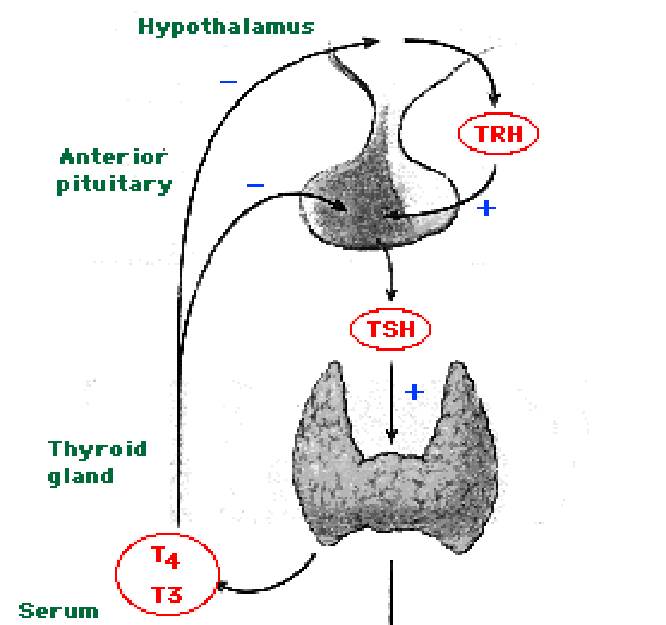
- GnRH (gonadotropin releasing hormone) which stimulates gonadotropes of the anterior pituitary to release LH and FSH.
- TRH (thryroid releasing hormone) which stimulates thyrotropes and mammotropes (lactotropes) of the anterior pituitary to release TSH and PRL.
- PIF (prolactin inhibitory factor, dopamine) which inhibits lactotropes (mammotropic cells) of the anterior pituitary from releasing PRL.
- CRH (corticotropin releasing hormone) which stimulates corticotropes of the anterior pituitary (adenohypophysis) to transcribe POMC and release ACTH and beta-LPH.
- GHRH (growth hormone releasing hormone) which stimulates somatotropes of the anterior pituitary.
- SST (somatostatin) which inhibits somatotropes and thyrotopes of the anterior pituitary (adenohypophysis) to release GH and TSH.
- The hypothalamus also contains two more nuclei that produce two other hormones that are delivered directly to the posterior pituitary (neurohypophysis) through the axons of the neuron cells that produce the hormones.
- The supraoptic neucleus produces vasopressin (ADH, AVP) which acts on the collecting ducts of the kidney (think AQ2).
- The paraventricular nucleus produces oxytocin which acts on the mammary glands (myoepithelial cells) and uterus (smooth muscle cells, contractions).
- Oxytocin may be associated with increased generocity.
Origin, organization, and secretion of the pituitary gland (hypophysis)
- The hypophysis (pituitary gland) is located below the brain in the sella turcica, a cavity of the sphenoid bone.
- The hypophysis has two regions: the adenohypophysis (anterior pituitary) and the neurohypophysis (posterior pituitary).
- The adenohypophysis originates from the oral ectoderm.
- This makes sense because it has "ad" "deno" which means "toward the teeth".
- The neurohypophysis originates from the brain.
- This makes sense when one recalls that it is the posterior pituitary (neurohypophysis) into which neurons from the hypothalamus directly connect and directly release hormones.
- Also, knowing that the neurohypophysis (posterior pituitary) originates from brain tissue makes sense because one of the hormones released by the posterior pituitary (neurohypophysis) has a very neurotransmitter-like role: oxytocin causes muscle contraction.
- The adenohypophysis originates from the oral ectoderm.
- The pituitary gland's two divisions have several components:
- The anterior pituitary consists of the pars distalis and the pars tuberalis.
- The pars intermedia separates the two functional units.
- The posterior pituitary (neurohypophysis) is made up of the pars nervosa and the infundibular stalk.
- The infundibulum is the combination of the infudibular stalk and the pars tuberalis, from the neurohypophysis and adenohypophysis, respectively.


- Embryology of the pituitary gland
- Recall that the adenohypophysis arises from the oral ectoderm.
- The oral ectoderm (roof of the mouth) grows caudally, forms Rathke's pouch, and eventually separates the pouch from the oral ectoderm.
- Recall that this caudal pouching of the oral ectoderm generates the pars tuberalis, the pars distalis, and the pars intermedia.
- Recall that the neurohypophysis arises form the brain tissue.
- The neuroectoderm (floor of the diencephalon) grows caudally, forms a stalk, and remains attached to the brain tissue of origin.
- Recall that this caudal stalk formation generates the pars nervosa, the median eminance, and the median eminence.
- Recall that the adenohypophysis arises from the oral ectoderm.

Adenohypophysis (anterior pituitary)
- The adenohyophysis is composed of the pars distalis, the pars tuberalis and the pars intermedia.
- The pars distalis is the same as the anterior lobe.
- The pars distalis (anterior lobe):
- The pars distalis is composed of fibroblast generated reticular fibers that support hormone-generating epithelial cells and a rich bed of fenestrated capillaries.
- Cells of the pars distalis can be classified by the way the stain: basophilic, acidophilic, and chromophobes.
- Acidophilic cells: somatotropes and mammotropes (lactotropes).
- Basophilic cells: gonadotropes, croticotropes, and thyrotropes
- Chromophobic cells: stem cells, degranulated cells that would otherwise be chromophilic (see acidophilic and basophilic).
- Differentiating cell types is not possible with light microscope, only by trasmission electron microscopy can these hormone producing cells be differentiated.

- The pars tuberalis:
- This is a funnel shaped structure that surrounds the infudibular stalk of the neurohypophysis.
- Most cells of the pars tuberalis are basophilic.
- The pars intermeida:
- The pars intermedia the lumenal remnant of the pouch part of Rathke's pouch.
- The pars intermeida separates the pars distalis of the adenohypophysis and the pars nervosa of the neurohypophysis.
- Colloid-filled cysts fill the pars intermedia.
Neurohypophysis (posterior pituitary)
- The neurohypophysis is derived from the neuroectoderm and contains two major regions: the pars nervosa and the infundibular stalk.
- The neurohypophysis contains nerve cells and glial cells (pituicytes).
- The pars nervosa:
- The pars nervosa contains fibroblasts, pituicytes, mast cells and neurons.
- The neurons arise from the paraventricular and supraoptic neuclei where oxytocin and vasopressin are made, respectively.
- These neurons are atypical in that they do not synapse at their distal axons.
- The hormones released by these neurons are stored in granules (called Herring bodies or neurosecretory bodies) at the distal aspect of the axon.
- Herring bodies can be identified under light microscopy.
- The infundibular stalk
- The infundibular stalk, like the pars nervosa, contains atypical nerve axon endings that release hormones.
- The neurons of the infundibular stalk release their hormones into the hypothalamus-pituitary portal system and affect the cells of the anterior pituitary.
Pituitary portal system
- There are superior, middle, and inferior hypophyseal arteries that service the adenohypophysis.
- There are really 4 main components to the portal system: primary and secondary capillary beds, long veins and short veins.
- The primary capillary bed arises from the superior hypophyseal artery and resides around the median eminance.
- At the primary capillary bed, neurons of the hypothalamus dump hormones into the blood stream.
- The long veins connect the primary capillary bed to the secondary capillary bed.
- The secondary capillary bed resides around the adenohypophysis.
- At the secondary capillary bed, hormones from the hypothalamus exit to affect the cells of the anterior pituitary and hormones form the anterior pituitary (adenohypophysis) enter the blood stream.
- The inferior hypophyseal artery forms a capillary mesh at the neurohypophysis.
- The short veins connect the capillaries of the neurohypophysis to the secondary capillary bed of the adenohypophysis.
- It is unclear if there is a particular function associated with this connection between the neurohypophysis and adenohypophysis.
Clinical correlate: growth hormone deficiency
- Growth hormone deficiency results in low levels of GH release and therefore low levels of IGF1, IGF2, and IGF-binding protein 3 from the liver.
- Decreased levels of IGFs results in decreased growth and stature and delayed physical maturation.
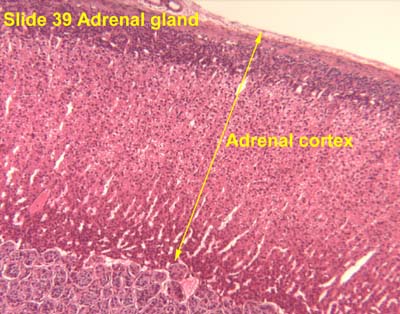
Origin, organization, and secretion of the Adrenal glands
- The adrenal glands sit atop the kidneys and have an outer shell and two functional compartments.
- The outer shell is made of dense connective tissue that sends septa into the center of the organ as trabechulae.
- The two functional units are the cortex and the medulla.
- The cortex and the medulla have different embryological origins and different functions and morphology.
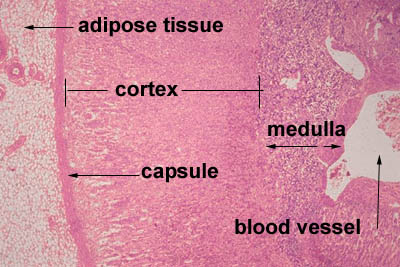
Adrenal cortex
- The adrenal cortex is derived from mesoderm (connective tissue progenitor).
- The adrenal cortex has three layers from superficial to deep: glomerulosa, fasciculata, and reticularis.
- GoFaRe: Glomerulsoa, Fasciculata, Reteicularis
- GomiFacoRea: glomerulus-mineralocorticoids, fasciculata-corticoids, reticularis-androgens.
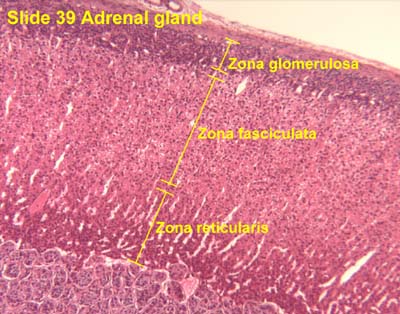
- The glomerulosa:
- The glomerulosa sits just below the adrenal capsule and is responsible for mineralocorticoid production.
- The glomerulosa layer is characterized by closely-packed, arched chords of columnar or pyramidal cells surrounded by capillaries.
- The glomerulus can be differentiated from the capsule because of increased cellularity, prominent, circular nuclei, prominent arches, less connective tissue (which usually stains bright pink).
- The fasciculata:
- The fasciculata sits just below the glomerulosa layer and is responsible for production of glucocorticoids and a small amount of sex steroids.
- The fasciculata is characterized by long chords of polyhedral cellls and fenestrated capillaries.
- The fasciculata can be differentiated from the glomerulosa by distinct change in from short, bulbous cellular collections to long chord-like cellular collections.
- The reticularis:
- The reticularis deepest (below the fasciculata) and is responsible for production of androgens which can also be converted to estrogens.
- Note that the reticularis mostly produces dehydroepiandosterone (a weak androgen) that can be converted into more potent androgens and then on to estrogens.
- The reticularis can be differentiated from the fasciculata by less organized cellular collections, more eosinophilic staining (pinker, think about the granules of norepi and epi),
- The reticularis deepest (below the fasciculata) and is responsible for production of androgens which can also be converted to estrogens.



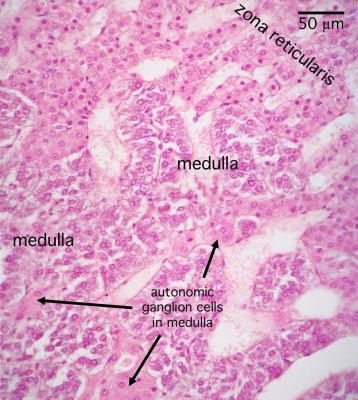
Adrenal medulla
- The adrenal meduall is derived from the neural crest and is responsible for making norepinephrine and epinephrine.
- This makes sense because norepi and epi are neurotransmitters.
- The medulla of the adrenal is composed of chromaffin cells which can be considered like post-ganglionic neurons.
- That is, preganglionic neurons synapse on chromaffin cells just like a pre-ganglionic neuron would synapse onto a postganglionic neuron.
- Chromaffin cells can either be norepinephrine producing or epinephrine producing and will have granules full of their labors.
- There is an association between the chromaffin's product and it's tissue location.
- Norepinephrine-producing neurons are found near medullary arteries.
- Epinephrine-producing neurons are found near cortical sinuses.
- Chromaffin cells stain lightly with euchromatin chunks visible in the nucleus.
- Cell density within the medulla is less than that of the cortex.



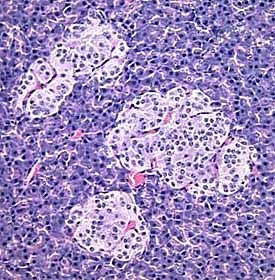
Origin, organization, and secretion of the Pancreas
- Recall that the pancreas is a mixed organ (exocrine and edocrine) with exocrine acinar tissue making up the primary structure.
- Recall that acinar of the exocrine tissue secrete gastric enzymes onto an epithelial duct system that enters the epithelium of the GI tract.
- The endocrine portion of the pancreas arises from endodermal tissue near the bile duct.
- The notes also say that the endocrine protion arises from epithelium of the gut.
- The islets of Langerhans are the endocrine portion of the pancreas.
- The islets of langerhans are surrounded by a capsule of reticular fibers that separate them from the exocrine tissue.
- There are four cell types in the endocrine islets of langerhans: beta, alpha, delta, and F / pp cells (by abundance).
- Beta cells make insulin.
- Alpha cells make glucagon.
- Delta cells make somatostatin.
- F / pp cells make pancreatic peptide.
- Islets of Langerhans are generally easily recognized as they stain a different shade or hue than the exocrine acinar tissue of the exocrine pancreas.



Origin, organization, and secretion of the Thyroid
- The thyroid gland originates from the foregut endoderm, near the base of the tongue.
- The thyroid gland sits anterior to the larynx in the cervical area.
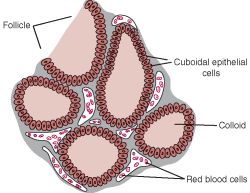
- The parenchyma of the thyroid gland is composed of epithelial cell funtional units called follicles.
- Parenchyma: "the functional part of an organ, as opposed to supporting tissue; the tissue making up most of the non-woody parts of a plant" per wiktionary
- Thyroid follicles have a simple epithelial ring of cells and a lumen within that is filled with colloid.
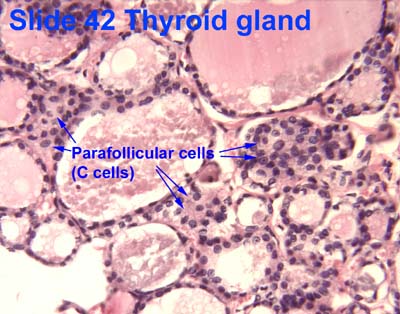
- Within or between the follicles can be found C cells (parafollicular cells) which produce calcitonin.
- Recall that calcitonin regulates serum calcium by decrease absorption at the gut, decreasing reabsorption at the bone, and increase excretion at the kidney.
- Thyroid hormones are T3 and T4.
- Iodide is required for production of T3 and T4.
- Production, storage, and release of thyroid hormones involves both endocrine and exocrine functions.
- Synthesis of T3, T4:
- Thyroglobulin made in RER, glycocylated in RER / golgi, and moved into the lumen.
- Iodide absorbed at basolateral surface (from blood) and oxidized at apical surface.
- Tyrosine residues of thyrogobulin iodinated at apical surface of follicular epithelial cells.
- Thyroglobulin pinocytized by follicular cells, fused with lysosomes, cut up to release T3 and T4.
- T3 / T4 released into blood at basolateral surface.

Origin, organization, and secretion of the Parathyroid glands
- The Parathyroid gland arises from the pharyngeal pouches.
- Like the adrenal glands, the parathyroid glands have a capsule with septa that run inward.
- The parathyroid gland is composed of two cell populations: chief cells and oxyphil cells.
- Chief cells:
- Chief cells produce PTH which serves to increase serum by increasing absorption at the gut (via increased activation of vitD to 1,25 vitD), increasing reabsorption at the bone, and decreasing loss at the kidney.
- Chief cells contain granules eosinophilic granules of PTH.
- Note that regulation of chief cell PTH release is an inhibition of inhibitoin mechanism: when serum Ca levels decrease, fewere Ca-receptors bind Ca (the ligand) causing a decrease in intracellular signaling and an increase of PTH release.
- Oxyphil cells:
- The function of oxyphil cells is unknown; however it is known that they arise during puberty.
- Oxyphil cells are larger than chief cells with an acidophilic cytoplasm and abnormally shaped mt.
- Oxyphil cells are often found in clusters at the center of the parathyroid gland or near the perimeter.




Primary hyperparathyroidism
- Primary hyperparathyroidism is a defect with the parathyroid itself causing an an elevation of PTH.
- Elevated PTH causes increased bone reabsorption, decreased stature, fractures, et cetera.
- Animals infused with Ca had more trabeculae, increased bone density, and shorter bone length.
- Giving PTH intermittently to post-menopausal women is associated with decreased risk of bone fracture.
- This makes sense when you understand that continuous administration of PTH causes bone loss yet intermittent PTH administration causes increases in bone mass.
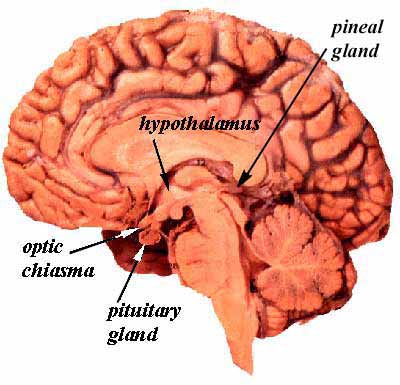
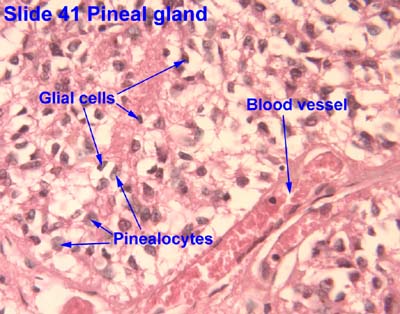
Origin, organization, and secretion of the Pineal gland
- The pineal gland arises from neuroectoderm from the floor of the diencephalon (just like the neurohypophysis).
- The pineal gland is pine-cone shaped and covered with connective tissue.
- This pine-cone shaped pineal gland is located in the posterior aspect of the third ventricle.
- The pineal gland contains pinealocytes, interstitial glial cells (like astrocytes).
- The pinealocytes produce melatonin and thus take part in daily rhythmicity.
- Rene Descarts explained human behavior and thought via the pineal gland because of its involvement in sensation, imagination, memory, and bodily movement.

Origin, organization, and secretion of the Diffuse Neuro-endocrine system
- We now know that many diffuse organs contain hormone-secreting endocrine cells that are important in physiological regulation of the body.
- Organs that have diffuse endocrine tissue include the heart, kidney, thymus, gut, and gonads.
- We often think of this diffuse set of endocrine cells as having primarily paracrine effects on nearby cells of the host tissue (like cardiomyocytes in the heart, etc.).
- An example of these diffuse endocrine cells...
Get duodenum and cardiac examples form audio.
Bone as an endcrine organ
- We are beginning to understand that bone is an important regulator of aspects of physiology.
- Two major signals are released by bone to affect physiology: FGF23 and uOCN.
- FGF23 is released by the bone and causes:
- Kidneys decrease phosphate (Pi) reabsorption resulting in decreased serium Pi.
- Kidneys decrease 1,25VitD activation resulting in decreased serum 1,25OH VitD.
- uOCN is released by the bone and causes:
- Pancreatic beta cells to increase insulin release resulting in decreased serum glucose.
- Adipocytes to increase adiponectin resulting in changes to glucose and fatty acid catabolism.
- Muscle to increase sensitivity to and uptake of glucose resulting in decreased serum glucose.
- Bone also releases osteocalcin which has been shown to be associated with poor fertility when deficient.
Lab
- started here on 03/28/11.
Female reproductive histology
Ovarian cycle
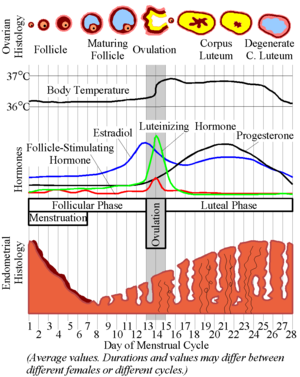
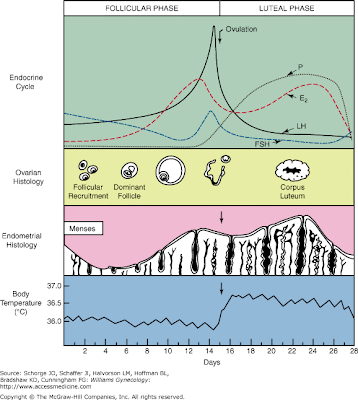
- The ovarian cycle describes the hormonal, anatomical, and reproductive changes that occur on a regular basis as the ovary generates a mature gamete for potential fertilization.
- The ovarian cycle coincides with the uterine cycle (which describes the hormonal and anatomical changes that conditions the reproductive tract for hosting an embryo).
- The ovarian cycle is primarily focused on the anatomical changes of the ovary (which contains the germ cells) and the changes in LH, FSH, estrogen, and progesterone.
Origin and fate of ovarian follicles
- Female gametes are generated from primordial germ cells in the ovaries, using a structure called the follicle.
- Ovarian follicles are composed of a germ cell (oocyte) surrounded by supporting cells (follicular epithelial cells).
- Ovarian follicle follicular epithelial cells include granulosa and theca cells.
- Primordial germ cells migrate originate from the yolk-sac (endoderm) and migrate to the genital ridge where the ovaries are developing.
- Note that this it makes sense that the germ cells have to migrate to the site of ovary development because they are of two different origisn: germ cells from yolk-sac endoderm and epithelium.
- Follicles undergo proliferation and reach their maximum number Follicles first develop at the genital ridges.
- There are approximately 3.5 million oocytes, 1 million oocytes, and 0.5 million oocytes at the fetal, neonatal, and puberty stages of development.
Ovarian anatomy
- The ovaries have cortex (outer) and medulla (inner) compartments.
- The cortex epithelium of the ovary is simple cuboidal epithelium.
- The cortex is the location of follicles surrounded by connective tissue and fibroblasts that make up the stroma.
- The medulla is the vascular core.


Ovarian follicle
- The follicle is a structure of follicular epithelia that surrounds the oocyte.
- Epithelial follicular cells proliferate throughout the follicular phase of the ovarian cycle.
- During the ovarian follicular phase mesenchymal cells will differentiate into theca cells to surround the follicle with an extra two layers.
- Theca cells of mesenchymal origin provide an inner endocrine layer of cells and outer vascular layer of cells.
- The endocrine layer of theca cells is called the theca interna; the vascular layer of theca cells is called the theca externa.
- The ovarian follicle has a specific anatomy of layers:
- The oocyte is at the center with a dense nucleolus, condensed chromosomes, many perinuclear mitochondria, and a fair amount of cytoplasm.
- The oocyte is surrounded by a dense layer called the zona pellucida (which will play an important role in fertilization)
- The zona pellucida is surrounded by follicular cells called granulosa cells which will be mitotic in the follicular phase
- Within the follicular cell population one may find a Call-Exner body which are collections of granulosa cell membrane with granulosa secretions within.
- The follicular cells are surrounded by a basement membrane (basal lamina).
- The basal lamina is surrounded by theca cells (from mesenchyme) which form two layers: theca interna and theca externa.
- Finally, the entire follicle with theca cell layers is surrounded by connective tissue of the ovary.




Stages of the ovarian follicle
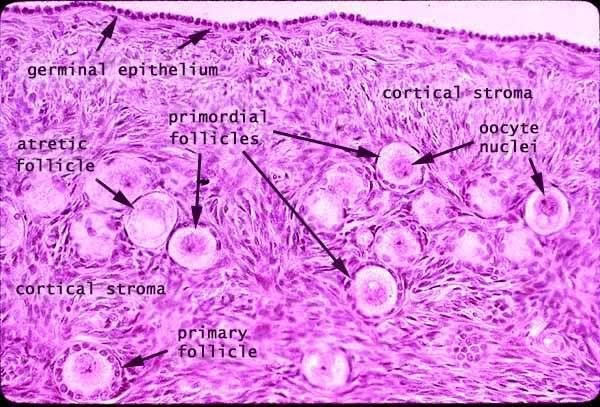
- Recall that the ovarian follicle includes the oocyte and surrounding supporting follicular epithelial cells.
- The follicle begins as a primordial follicle, undergoes changes that facilitate maturation of the oocyte.
- Changes to the follicle include proliferation of the epithelial cells (granulosa cells), formation of a basement membrane, formation of the zona pellucida, and formation of the thecal layers.
- There are 4 stages (for our purposes) of follicular development: primordial follicle, developing follicle, secondary follicle, mature follicle.
- Eventually the follicle ejects the oocyte in a process called ovulation.
- After ovulation the follicle has several potential fates: atresia and differentiation into a corpus luteum.
- Note that atretic follicles are characterized by a lack of cells but a circular shape that was once a follicle.
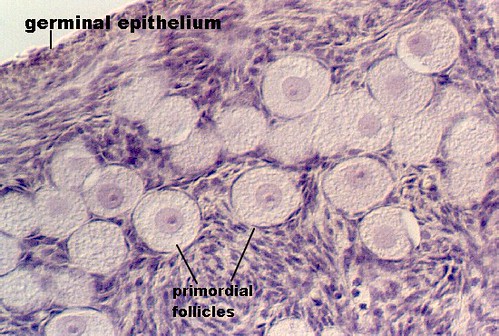
- Primordial follicle:
- The primordial follicle' contains a primordial germ cell and is surrounded by a single layer of follicular epithelial cells.
- Note that the follicular cells are squamous but are not yet considered granulosa cells.
- The primordial follicle is uniquely defined by having only a single layer of follicular epithelial cells.
- The germ cell of the primordial follicle is arrested in prophase of meiosis 1 and is therefore 4N with condensed, crossed-over chromosomes.
- Developing follicle:
- The developing follicle is uniquely defined by a basal lamina, a forming zona pellucida, multiple layers of granulosa cells and a theca interna.
- In the developing follicle, the follicular cells are cuboidal and have proliferated and differentiated into granulosa cells.
- Recall that theca cells arise from the stroma of the ovary--a source of mesenchymal cells.
- The zona pellucida is composed of glycoproteins and polysacchardies from both the granulosa cells and the oocyte.
- Note that communication to the oocyte can occur through cytoplasmic processes that extend form the zona pellucida into the oocyte.
- This connection occurs via gap junctions.
- Secondary follicle:
- Secondary follicles are also called atral and vesicular follicles.
- The secondary (vesicular, antral) follicle is uniquely defined by a developing antrum and a theca externa.
- Clearly this makes sense with names like antral follicle or vesicular follicle.
- Granulosa cells reach their mature state in the secondary follicle and serve several purposes:
- Granulosa cells communicate with the oocyte via gap junctions.
- Granulosa cells contribute to the zona pellucida (think glycoproteins and polysaccharides).
- Granulosa cells produce the follicular fluid that fills the antrum.
- Follicular fluid is filled with hormone binding proteins (like SHBG) and will thus become an important source of hormones for oocyte development and then pregnancy maintenance.
- Granulosa cells converte androstendione to estradiol (via aromatase).
- Granulosa cells secrete stroma-weakening factors to allow expansion of the follicle.
- A primary stroma-weakening factor is plasminogen-activator which converts plasminogen to plasmin (fibrinolysin, a trypsin-like enzyme) which cuts up fibrin.
- Granulosa cells secrete a meiosis-regulationg factors to inhibit movement from prophase 1 to metaphase 2 in the oocyte.
- Recall that the theca interna is considered the endocrine layer of thecal cells.
- Theca cells of the theca interna produce androstendione.
- Androstenedione from the theca interna cells is converted to estradiol by the granulosa cells.
- It is in the secondary follicle stage (antral stage, vesicular stage) that the oocyte reaches its mature size.

- Mature follicle:
- Mature follicles are also called Graafian follicles.
- The mature follicle is uniquely defined by the corona radiata and the cumulus oophorus.
- The corona radiata is a layer of granulosa cells that surrounds the zona pellucida of the oocyte along the antral aspect. The corona radiata is sometimes called the rim.
- The cumulus oophorus is a layer of granulosa cells that attaches the zona pellucida (and therefore the oocyte) to the membrana granulosa--the antral-surrounding layer of granulosa cells. The cumulus oophorus is sometimes called the stalk.
- Mature follicles are very large: can be over 1 cm!
- As a mature follicle, the oocyte progresses from prophase of meiosis 1 to metaphase of meiosis 2 and thus generates the first polar body.
- Note that having entered meiosis 2, the oocyte is called a secondary oocyte.
- Note that there are two physiological maturing processes occurring: the oocyte and the follicle but only the follicle maturity can be seen histologically.
- Graafian follicle is unlikely to be seen in lab because they are rare (one per month) and really big (size of a dime) and has a little oocyte in size (so it is unlikely that we would know it was a Graafian follicle even if it was sectioned).

http://t0.gstatic.com/images?q=tbn:ANd9GcR_5ajvKIDofXIvPSq_8CEqUgDbjlsBRtYNqKEDaGYemdfQoqPAtQ

- Deciding on which type of follicle you're observing:
- flattened follicular cells: primordial
- cuboidal folliclar cells (with a layer on either side--zona pellucida or basal lamina): developing
- antrum / fluid: secondary follicle
- no way to distinguish secondary from mature.

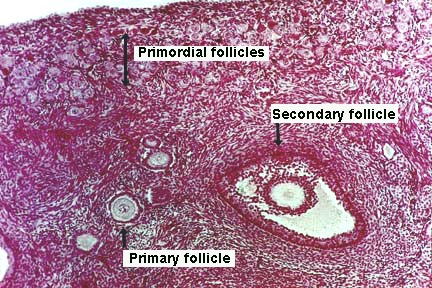
- Here is a good image (though it does not show a mature follicle):



Endocrine regulation of follicle maturation
- FSH from the anterior pituitary augments the maturation of follicles that are developing in the ovary.
- As one follicle becomes dominant, it produces follicular regulatory protein to inhibit growth of all other follicles.
- The other near-mature follicles undergo degeneration because of the follicular regulatory protein.
- The other primordial follicles are signaled to mature no more during this cycle.
- Recall that granulosa cells proliferate in the secondary stage of follicular development and that granulosa cells generate estrodiol from the androgens produced by interna theca cells.
- Increased estrogen inhibits FSH release at the pituitary thus stopping the growth of the follicle and allowing ovulation.
- Increased estrogen stimulates LH release at the pituitary thus commencing ovulation.
- Recall these classic images:

Ovulation
- Generally a single egg is released into from one of the two ovaries.
- As the granulosa cells of the secondary and mature follicle produce more and more estrogen, more and more LH is released from the anterior pituitary gland (adenohypophysis, pars distalis).
- Ovulation events include:
- Breakdown of the cumulus oophorus, thus the oocyte floats freely in the antrum and follicular fluid.
- Weakening of the ovarian stroma:
- Proteolytic enzymes like collagenase disrupt the stromal connective tissue.
- Granulosa cell connections weaken
- Local ischemia causes a pale spot on the surface of the ovary called a stigma.
- Follicular wall ruptures releaseing an oocyte with the corona radiata and zona pellucida surrounding.
The corpus luteum
- After the dominant ovarian follicle undergoes ovulation, the follicle may degenerate or become a temporary endocrine structure called the corpus luteum.
- The corpus luteum is a differentiation of the granulosa and theca cells in response to LH signaling.
What determines if the follicle becomes a corpus luteum? Something made by the placenta?
- LH causes granulosa cells to become granulosa lutein cells.
- Granulosa lutein cells develop the morphology of a secretory cell and actively produce progesterone.
- Note that the production of progesterone by the granulosa lutein cells is necessary for implantation of the embryo.
- Progesterone literally means "keep gestation going hormone": pro + gest + erone.
- LH causes theca cells to become theca lutein cells.
- Theca lutein cells continue to produce androsteindione and other estrogen precursors.
- Note that a clot within the follicle vascularizes to perfuse the granulosa lutein cells that make up the center of the corpus luteum.
- The clot is generated at ovulation.
- If pregnancy does not occur, the corpus luteum is called the corpus luteum of menstruation.
- The corpus luteum of menstruation is maintained as long as LH is present.
- Recall, however, that the corpus luteum produces progesterone and that progesterone has an inhibitory effect on LH release by the pituitary.
- Therefore, progesterone from the corpus luteum is self limiting.
- That is, the corpus luteum will bring about self-demise via progesterioen inhibition of pituitary-LH unless chorionic gonadotropin is generated by the placenta.
- Without pregnancy, the corpus luteum lasts 10-14 days.
- If pregnancy occurs, the corpus luteum is called the corpus luteum of pregnancy.
- Recall that trophoblasts of the placenta produce chorionic gonadotropin which maintains the corpus luteum (even though LH drops because of high progesterone levels).
- hCG is the hormone used to test for pregnancy.
- Granulosa cells of the corpus luteum of pregnancy produce relaxin which has a smooth-muscle relaxing effect (histo says "during parturition", wikipedia says "during gestation").
- Relaxin opposes the pro-parturition actions of oxytocin; that is, it keeps the smooth muscle of the uterus relaxed.
- Relaxin targets the fibrocartilage of the pubic symphysis to increase articulation.
- Note that physio notes say that the role of relaxin in pregnancy is unclear.

Follicular atresia
- Follicles can undergo atresia from any stage of follicular development (primordial, developing, secondary, or mature).
- Follicular atresia generates a long-lasting, scar-tissue structure called the corpus albicans.
- Note that the larges of the coprus albicans found in an ovary likely arose from a previous corpus luteum.
- The zona pellucida remains (bright pink) and a wavy line (the basement membrane, called the glassy membrane).
- The largest atretic artifact, though, is the corpus albicans (the white body).

The uterine tubes
- The uterine tubes conduct the mature female gamete from the ovary to the uterus and are the site of fertilization.
- The uterine tubes can also be called oviducts or Fallopian tubes.
- The uterine tubes are muscular tubes that extend from the ovary on the posterolateral wall of the abdomen to the medioventral aspect of the abdomen and the lateral aspect of the uterus.
- The Fallopian tubes are approximately 12 cm long.
- There are four major sections to the oviducts from the ovary to the uterus: infundibulum, ampulla, isthmus, and interstitial segments.
- These are hard to identify so we will not be asked to identify the region of the oviduct.
- The infundibulum has fimbriae and "bears osteum.
What does "bears osteum" mean?
- The ampulsa is the dilated intermediate segment of the oviduct.
- The isthmus is the media 1/3 of the Fallopian tube.
- The interstitial segment pierces the uterine wall.
- As the oviducts progresses distally, there are fewer involdings.


http://www.jci.org/articles/view/29424/files/JCI0629424.f1/medium
Layers of the oviduct
- Like other epithelial tracts there are four major layers to the oviduct (from inner to outer): mucosa, lamina propria, muscularis, and serosa.
- Secretions from the oviduct promote sperm activation.
Does this refer to capacitation?
- Mucosa of the oviduct:
- The mucosa of the oviduct is comprised of columnar, ciliated epithelial cells.
- These columnar cells are secretory and are called Peg cells.
- Estrogen (from the corpus luteum) increases the height of the columnar cells.
- Progesterone (from the corpus luteum) increases the ciliary action of the columnar epithelial cells of the mucosa.




- Lamina propria:
- The lamina propria of the oviduct is highly vascularized.
- Fimbriae are especially concentrated with smooth muscle and become highly active around ovulation.

- Muscularis:
- As with so many muscularis layers, there is an inner circular and outer longitudinal layer.
- The two layers of the muscularis are interwoven.
- There is a peristaltic contraction movement toward the uterus.
- Note that one is unlikely to differentiate two layers in lab.

- Serosa:
- The serosa is a true serosa because it is lined with mesothelium.



- stopped here on 03/28/11.
- started here on 03/30/11.
The uterus
- The uterus is comprised of four parts (proximal to distal along the genital tract): the fundus, body, isthmus, and cervix.
- The fundus is the rounded superior end.
- The body is the main portion of the uterus.
- The isthmus is the constricted middle portion.
- The cervix is the cylindrical portion that projects into the vagina.
Layers of the uterine wall
- Like the oviducts and other epithelial tracts, there are four tissue-type layers to the uterus which make up three functional layers of the uterus.
- The tissue layers are (inner to outer): mucosa, smooth muscle, serosa, and adventitia.
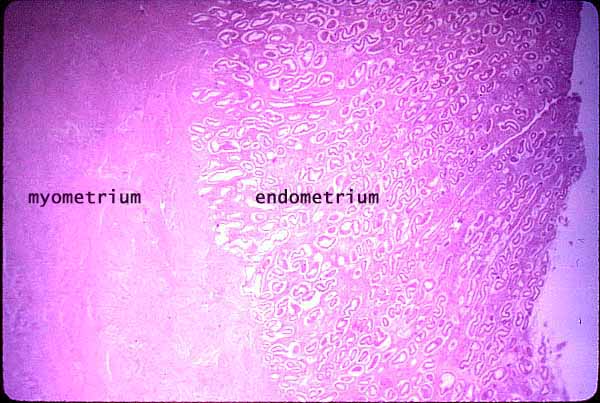
- The functional layers are (inner to outer): endometrium (think implantation), myometrium (think contraction), and epimetrium.
- The endometrium is composed of mucosa.
- The myometrium is composed of smooth muscle.
- The epimetrium is composed of serosa and adventitia and is a form of mesothelium as one would expect to cover surface of organs that faces the inside of the abdomenal cavity.
- Note that the cervix is histologically distinct from the rest of the uterus; we will revisit this.
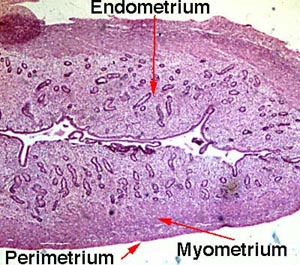





The uterine cycle
- Recall that the ovarian cycle describes the hormonal, anatomical, and reproductive changes of the gonads while the uterine cycle describes the hormonal and anatomical changes to the genital tract (including the uterus).
- The uterine cycle is also called the endometrial cycle as it is the endometrial layer of the uterus that undergoes most of the changes during the uterine cycle.
- From physio notes:
- The phases of the menstrual cycle can also be described by the changes to the uterus (the reproductive tract).
- The uterine cycle of menstruation has four stages: the proliferative stage, secretory stage, ischemic stage, and menstrual stage.
- The proliferative stage is characterized by endometrium hypertrophy and formation of spiral arteries.
- Recall that the uterine proliferative stage occurs during the ovarian follicular phase.
- So as the ovary is maturing its follicle, the uterus is regenerating it's surface (where the egg will implant) and increasing vascular access to the surface.
- The secretory stage is characterized by coiling of glands, secretion of mucus, tortuous arteries, and peak thickness of the endometrium.
- Recall that the uterine secretory stage occurs during the ovarian luteal phase.
- So, as the ovary has shed an ovum and is now increasing hormone production via the corpus luteum, the uterus is using glands and arteries of the uterus to modify the uterine microenvironment to the optimal conditions for egg implantation.
- The ischemic stage is characterized by arterial constriction, decreased blood flow, and increased prostaglandins.
- Recall that the ischemic stage occurs during the ovarian menses phase.
- So as the ovary has reached its lowest levels of hormone production, the uterus is decreasing nutrition to the endometrium and allowing the mucosa to undergo necrosis by ischemia.
- The menstrual stage is characterized by desquamation of the endometrium.
- Recall that the menstrual stage occurs during the ovarian menses phase.
- So as the ovary has reached its lowest levels of hormone production, the uterus is shedding its endometrium.
Uterine vasculature
- The primary changes of the uterine cycle are to the vasculature of the endometrium.
- Recall that the uterus is suspended from the lateral walls of the abdomen by the broad ligament which also serves to carry the vasculature of the uterus.
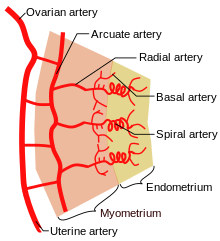
- The uterus is supplied by arcuate arteries that run along the myometrium layer and by radial arteries that cross into the endometrium.
- The radial arteries give off straight (basal) arteries that supply the endometrium basalis.
- Note that the endometrium is divided into two layers: the endometrium basalis is a constant, mostly unchanging layer while the endometrium functionalis cycles through generation (proliferation) and shedding.
- There is no structural marker to distinguish between the basalis and the functionalis of the endometrium.
- Spiral (coiled) arteries are heavily muscular, generated during the endometrial cycle, and bridge the radial arteries into the endometrial functionalis.
- Smaller arteries also exist off of the straight (basal) and spiral (coiled) arteries, including nutrient arterioles / capillaries, and venous sinusoids.
- Of special note are the vascular structures nearest the lumen of the uterus called lacunae.
Uterine endometrium
- Recall that the endometrium of the uterus contains the mucosa layer (of the four common epithelial layers: mucosa lamina propria, muscularis, and serosa).
- The endometrial mucosa contains uterine glands.
- Uterine glands are tubular with many branches.
- Uterine glands contain both ciliated and non-ciliated cells.

Histological changes in the uterine cycle
- Recall that there are four stages to the uterine cycle: menstruation, the proliferative stage, the secretory stage, and the ischemia stage.
- Note that we start with menstruation because in the ovary a new follicle is beginning to mature (follicular phase) so it seems like a good place to call the "start" in the gonads.
- The phases are divided over approximately 28 days: menstruation (days 1-5), proliferation (6-15), secretion (16-17), ischmia (18-28).
- Menstruation:
- Recall that during menstruation, the ovary is producing very little estrogen / progesterone and a follicle is in the beginning of its maturation.
- During menstruation, the endometrium functionalis is shed (secondary to ischemia).
- Note that the base of the uterine glands remain visible in the endometrium basalis.

- Proliferative stage:
- Recall that during the proliferative stage, a follicle in the ovary is maturing to secondary and Graafian stage and producing more and more estrogen and progesterone.
- The proliferative stage is driven by estrogen produced by the developing follicle.
- The proliferative stage is characterized by resurfacing of the endometrium through epithelial and stromal proliferation.
- This resurfacing requires the lengthening of the uterine glands and is accompanied by coiling of the glands.
- In addition to gland coiling, spiral arteries develop in the thickening endometrium.
- Cells of the proliferative endometrium accumulate glycogen.
Why accumulate glycogen? Probably for nourishment of the rapidly proliferating cells. I think probably to prep for the secretory phase when glycoproteins are secreted.
- Secretory stage:
- Recall that during the secretory stage the ovary has generated a corpus luteum and is producing estrogen and progesterone.
- Recall that the secretory stage takes place after ovulation and lasts until the corpus luteum degenerates or pregnancy is terminated.
- The secretory stage is driven by progesterone from the corpus luteum.
- The secretory stage is characterized by release of glycoprotein-rich products, swelling and torture of the glands and spiral arteries, and accumulation of fluid in the stroma of the endometrium.
- Ischemia:
- Recall that during the ischemic stage the ovary is seeing low hormone levels and the degeneration of the corpus luteum.
- Note that the ischemic stage does not occur when pregnancy is initiated by implantation and the placenta maintains estrogen and progesterone levels.
- The ischemic stage is characterized by constriction of the coiled arteries, stromal fluid loss, and lymphocyte / macrophage cell invasion.
- Recall that progesterone inhibits prostaglandins and that prostglandins are potent vasoconstrictors.
- When the corpus luteum degenerates and progesterone levels drop, local prostaglandins are released into the endometrium, the vessels constrict, and blood flow is arrested causing ischemia.
- The coiled arteries dilate and constrict intermittently which causes ischemia, cell lysis, a weakened stroma, bursting vessles, and debridement of the functionalis.
- The arteries both restrict oxygen (constriction) to cause cell death but also to flush away the dead tissue (dilation).
Uterine cervix
- As mentioned before, the cervix is histologically distinct from the rest of the uterus.
- Note that the vasculature of the cervix is stable; that is, no part of the vasculature of the cervix changes throughout the month.
- The cervical mucosa:
- The cervical mucosa of the uterus is not shed at menstruation like it is along the rest of the uterus.
- The cervical myometrium:
- The cervical myometrium has less smooth muscle and abundant collagenous connective tissue with elastic fibers.
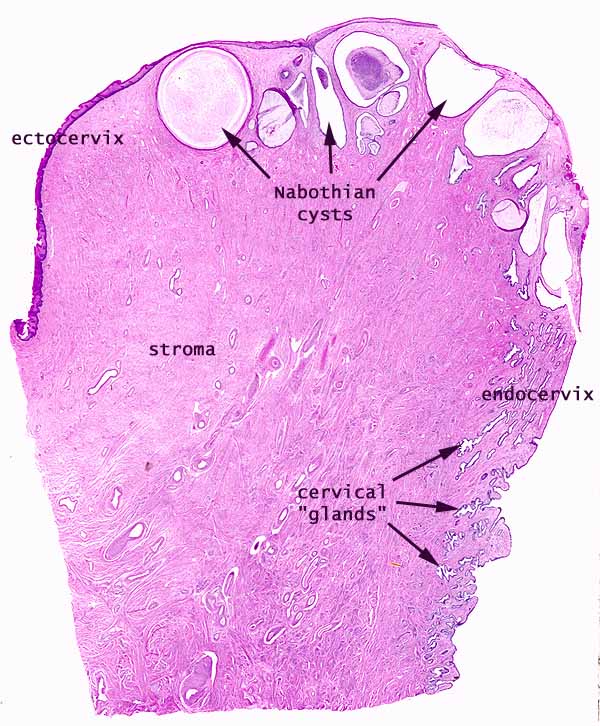
- The cervical endometrium:
- The cervical endometrium has denser stroma, simple columnar epithelium, branched, dilated, cyst-forming glands, and longitudinal mucosal folds called plicae (plicae palmatae).
- Cervical glands can form cysts called Nabothian cysts.
- These Nabothian cysts are not usually pathological.
- The cervical endometrium has denser stroma, simple columnar epithelium, branched, dilated, cyst-forming glands, and longitudinal mucosal folds called plicae (plicae palmatae).
- The cervical mucus:
- The cervical mucus changes throughout the uterine cycle.
- Mid way through the cycle (think ovulation and sperm-friend environment) the mucus is watery, contain lysozyme (bacterioalcidal), and promotes sperm motility.
- This sperm-friendly mucus is estrogen-stimulated.
- Late in the uterine cycle (think corpus luteum and potential implantation) the mucus is viscous and progesterone-stimulated.
- During pregnancy the mucus is particularly thick (think lots of progesterone) and thus protective of the fetus.
- One may look for the loss of this dense mucus plug as a sign that parturition is commencing.
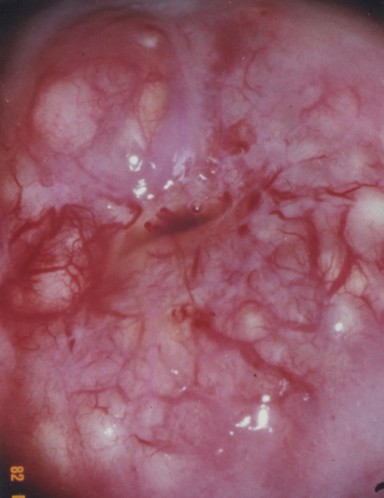


The ectocervix
- The ectocervix is the juncture between the cervix and the vagina.
- The ectocervix is also called the portio vaginalis.
- At the ectocervix the epithelium changes from columnar (cervix) to stratified squamous (vagina) abruptly.
- The ectocervix is a common site for cell growth abnormalities like dysplasias, neoplasias, and invasive carcinomas.
- Hence we do Pap smears from this area of the uterus.
- Normal ecotcervix:


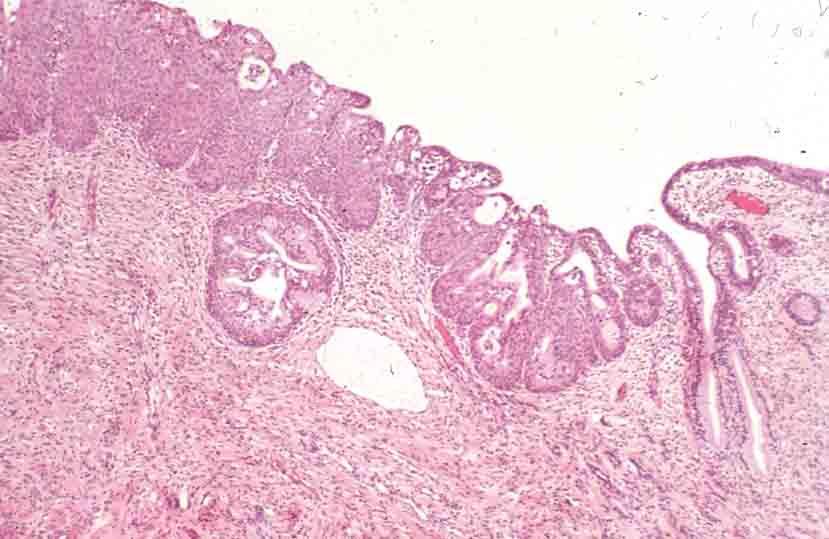
Vagina
- The vagina has the standard four layers for an epithelial tract: mucosa, lamina propria, muscularis, and serosa (adventitia).
- The mucosa is characterized by stratified, squamous, non-keratinized epithelium.
- The epithelial cells of the vagina--like those of the uterus--accumulate glycogen upon estrogen signaling.
Why the glycogen?
- The vaginal lamina propria has no glands, patches of lymphocytes, and can have folds.
- Recall, however, that the uterus does have glands in the lamina propria.
- The vaginal muscularis has interlacing bundles of smooth muscle.

.jpg)


Mammary glands
- The mammary glands are made of a compound tubuloalveolar system; that is, there are alveoli that take part in the secretory component and there are ducts that take part in the transport component.
- A group of about 20 glands forms a mammary lobule.
- Secretory component:
- Milk is generated by cuboidal epithelial cells arranged in alveoli.
- The alveoli develop as outgrowths of the ducts.
- Alveoli are surrounded by myoepithelial cells (that, upon oxytocin signaling, contract to move milk along the ducts).
- The myoepithelial cells arise from the cuboidal epithelial cells.
- Plasma cells are found in and around the alveoli in order to generate IgA.
- Milk is generated by cuboidal epithelial cells arranged in alveoli.
- Mammary ducts:
- The epithelium that lines the ductule system is stratified cuboidal.
- From the alveoli milk runs through lactiferous ducts to lactiferous sinuses and on to dilated reservoirs.
- There is a significant amount of smooth muscle between the ducts and sinuses.
- Most breast cancers arise from lactiferous duct cells.






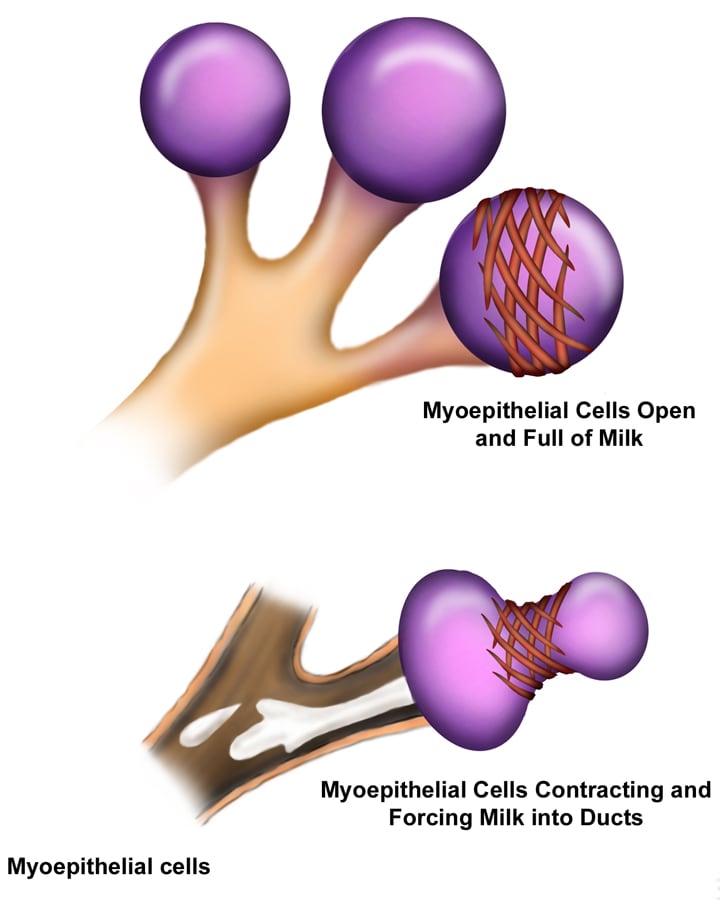
Mammary glands at puberty
- Before puberty, the lactiferous ducts have formed but no alveoli exist.
- When estrogen increases at puberty (and prolactin is present), alveolar buds develop and regress with each ovarian cycle.
- Note that these alveolar buds are not full alveoli.
Mammary glands in pregnancy
- Upon pregnancy, estrogen is found at high levels along with prolactin, placental lactogen, and progesterone and therefore the alveolar ducts and alveoli fully develop.
- There are then two stages of milk production; lactogenesis is the initial production of milk while galactopoiesis is the continued production of milk.
- Lactogenesis is regulated by estrogen, progesterone, and prolactin.
- This makes sense because estrogen and progesterone increase throughout pregnancy and once progesterone and estrogen drop (at parturition), prolactin has its most potent effect.
- Galactogenesis is maintained by prolactin and oxytocin.

Breast milk
- The first milk generated is called colostrum; colostrum is lactoprotein- and immunoglobulin- rich and lipid-deficient.
- Lactation can generate 1100 to 2100 ml every day.
- Note that physio said 800-1200 ml / day.
- Recall that the cuboidal epithelial cells of the alveoli generate milk.
- These cuboidal epithelial cells use several secretion mechanisms to release their products.
- Merocrine secretion is used to secrete casein, alpha-lactalbumin, and PTH-RP (protein, basically).
- Apocrine secretion is used to secret TAGs and cholesterol.
- Exocytosis is used to secrete lactose.
- Recall that most sugar movement in the body uses channels (usually Na linked); lactose requires a exocytosis.
- Transcytosis (from adjacent plasma cells) is used to secrete dimeric IgA.
Regulation of milk let-down
- Milk "let-down" is the release of milk from the alveoli to the ductule system.
- Let down is stimulated by suckling at the nipple which stimulates an afferent neuronal signal to the posterior pituitary (neurohypophysis) where neurons release an efferent hormonal signal (oxytocin) that stimulates the myoepithelial cells to contract and force milk distally through the tract.
- Note that afferent CNS signals also stimulate dopamine inhibition such that prolactin is increased which stimulates the cuboidal epithelial cells of the alveoli to increase milk production.
- Note that multiple neuroendocrine factors have been found besides oxytocin to relax the smooth muscle sphincter between the lactiferous ducts and the lactiferous sinuses.
Summary
- FSH stimulates follicle development in the ovary.
- Estrogens increase as the follicle develops and:
- inhibit FSH at the pituitary
- stimulate LH at the pituitary
- LH surge stimulates ovulation
- LH promotes corpus luteum formation
- Corpus luteum produces progesterone and estrogen
- Estrogen causes uterine proliferation phase
- Progesterone causes uterine secretory phase
- Progesterone inhibits LH
- Without implantation:
- Corpus luteum degenerates for lack of LH
- Progesterone and estrogen levels decrease
- Menstruation
- With implantation:
- Chorionic gonadotropin maintains corpus luteum (maintains estrogen and progesterone)
- Uterus remains in secretory phase for pregnancy.
- stopped here on 03/30/11.
- started here on 04/06/11.
- State wide exam:
- Previously the NBME exam had nothing to do with our histo course; there was lots of CMB, etc.
- So now they make a customized NBME.
- 100 questions
- 2.5 hours (150 minutes)
- Does not cover special senses.
- We are the last of the centers to take the NBME exam.
- How to study: use the high-yield materials; some have not studied and done just fine.
- Review session on Wednesday before the exam.
Male reproductive
Anatomy review
- The testes are egg-shaped organs covered with a cartilagenous capsule called the tunica albuginea.
- The testes are divided into lobules; lobule division is incomplete and achieved by the connective tissue septae.
- There are approximately 250 lobules in the testis.
- Each lobule has one or several seminiferous tubules.
- The mediastinum testis is where the vessels (blood and lymphatics), nerves, and efferent duct enter and exit the testis.
- The mediastinum testis lies at the posterior aspect of the testis.
- Note that the mediastinum testis is connective tissue while rete testis is a collecting tubule tissue.
- Seminiferous tubules are blind ended, highly coiled, and lined with spermatic epithelium.
- It is within the seminiferous tubules that spermatogenesis takes lace.
- The tunica propria is the outer wall of the seminiferous tubule and is made of smooth muscle and fibroblasts.
Seminiferous epithelium
- The seminiferous epithelium (the inside of the seminiferous tubule, recall) is a stratified epithelium and is responsible for generating male gametes.
- There are multiple types of spermatogonia in the seminiferous epithelium: type A and type B.
- Type A spermatogonia are stem cells.
- Type B spermatogonia are highly mitotic progenitor cells.
- Type B spermatogonia are connected via cytoplasmic bridges which help synchronize maturation of developing spermatozoa.
- Note that we will not differentiate between type A spermatogonia and type B spermatogonia in lab.
- Spermatids remain in close physical contact with the Sertoli cells throughout development.

Spermatogenesis
- Recall the order cell names in spermatogenesis: type A spermatogonia -> type B spermatogonia -> primary spermatocyte -> secondary spermatocyte -> spermatid -> spermatozoa.
- Primary spermatocytes are in the prophase of meiosis 1 and stick around for 20 days.
- Secondary spermatocytes are relatively short-lived.
- Note that spermatids shed their residual bodies as they become spermatozoa.
- Recall the order of divisions in spermatogenesis: mitosis, mitosis / differentiation, meiosis 1, meiosis 2, differentiation.
- Note that mitosis from type A spermatogonia to type B spermatogonia will maintain the stem cell population and will occur more than once such that many type B spermatogonia are generated.
- As spermatogonia develop, they move from the basal compartment to the adlumenal compartment.
- We call this adlumenal movement.
- Cells of the basal compartment: type A and type B spermatogonia, primary spermatocytes
- Cells of the adlumenal compartment: secondary spermatocytes, spermatids, spermatozoa
- Cells and processes: type A spermatogonia undergo mitosis to become ... type B spermatogonia undergo mitosis (and differentiation) to become ... primary spermatocytes undergo meiosis 1 ... secondary spermatocytes undergo meiosis 2 ... spermatids undergo morphologic modification (differentiation) ... spermatozoa.
- Spermatocytogenesis includes all the steps that generate an increasing number of cells (that is, type A spermatogonia through generation of secondary spermatocytes); this makes sense because of the name "cyto" = cell and genesis = "origin of".
- Spermiogenesis is the converse of spermatocytogenesis: spermeiogenesis is the maturation of existing cells into spermatozoa (from the secondary spermatocyte stage to the spermatozoa stage).
- It takes 60-70 days for spermatogonia to progress to spermatozoa.



Mitosis and Meiosis
- Recall that mitosis occurs when one 2N (diploid, like most cells of the body) cell makes a copy of the chromosomes (making it temporarily 4N--tetraploid) and divides the two copies up between two cells; both daughter cells are 2N.
- Recall that meiosis occurs when one 2N (diploid) cell makes a copy of the chromsosomes (making it temporarily 4N--tetraploid) and divides the two copies up between two cells--twice; all four daughter cells are 1N (haploid).
- So, primarly spermatocytes are the initial cell, secondary spermatocytes are the first generation of meiosis daughters, and spermatids are the second generation of meiosis daughters.
- That is, secondary spermatocytes are the result of meiosis 1 (2N->4N->2N) and spermatids are the result of meiosis 2 (2N->1N).
Spermiogenesis
- Recall that spermiogenesis occurs on existing cells (spermatids) and does not generate any new cells.
- Spermiogenesis is characterized by morphological changes to the spermatid, that is the specialization / differentiation of the spermatid into the spermatozoa:
- Loss of cytoplasm
- Condensation of genetic material and nucleus
- Formation of the acrosome
- Formation of the axoneme
- Spermiogenesis has 3 phases: golgi phase, acrosomal phase, maturation phase.'
- We will not be asked to identify the phase of a developing spermatid.
- Golgi phase:
- The golgi phase generates the polarization of the cell; it is called the golgi phase because the golgi becomes the acrosome.
- The enzymes like hyaluronidase and trypsin-like protease accumulate at one pole of the nucleus in a vesicle (which will become the acrosome).
- This makes sense because upon greeting an oocyte, the spermatozoa will use the enzymes within the acrosome to digest away the shell of the oocyte so it can fertilize.
- The centrioles (which will become the axoneme) migrates to the opposite pole as the enzyme vesicle.
- Acrosomal phase:
- The acrosomal phase is characterized by ... the development of the acrosome.
- The vesicle of enzymes flattens out over one pole of the nucleus.
- The cell rotates such that the axoneme faces the lumen.
- Maturation phase:
- The maturation phase is characterized by motile apparatus development and the capacity to fertilize.
- The cell body is shed, generating the residual body; upon shedding, the sperm are released into the lumen of the seminiferous tubule.
- Note that the sertoli cells clean up the residual bodies.
- Note that during the maturation phase, the spermatids are not yet motile or fertile.
- Though they may wiggle a little bit.
- Note that we can distinguish four parts of the spermatazoa: head, middle, principle piece, and end piece.
- The Head contains the nucleus and acrosome.
- The middle piece contains the mitochondria.
- The principle piece is primarily axoneme.
- The end piece is only the very last portion of the axoneme.

Sertoli cells
- Recall that Sertoli cells function as supporting cells for germ cells that generate spermatozoa.
- Sertoli cells condition the microenvironment for gamete production.
- Recall that Sertoli cells are physically close to the developing gametes.
- Sertoli cells are a tall, columnar epithelial cell.
- Sertoli cells are characterized by being tall, columnar epithelial cells with a large, indented euchromatic nucleus, and lots of eosinophilic cytoplasm.
- Neighboring Sertoli cells within a region have gap junctions which suggest that Sertoli cells are coordinated within their region.
- Coordination through gap junctions would explain the fact that different regions of the seminiferous epithelium (which includes sertoli cells and spermatogonium) are at different stages of spermatid development. (That is, while one place may have a batch of spermatids going through morphological changes to be come spermatozoa, the neighboring region could have a group of primary spermatocytes undergoing meiosis 1 to generate secondary spermatocytes.)
- Recall that it takes about 70 days for a spermatogonium to generate spermatozoa.
- stopped at minute 31.
Blood testis barrier
- It is important that the developing spermatozoa be kept separate from the blood supply because antibodies native to the pt can attack the developing spermatozoa because of immunologically active proteins generated through chromosomal recombination of meiosis 1.
- The capillaries of the testes are fenestrated; such a capillary provides little to know barrier against antibodies.
- Sertoli cells of the seminiferous epithelium form tight junctions between one another to keep immunoglobulins in the blood from entering the lumen of the tubule.
- Note that these tight junctions of the Sertoli cells are on the lumenal side of the spermatogonia.
- These tight junctions define the two compartments: basal compartment and adlumenal compartment.
- Recall that spermatogonia through the primary spermatocytes are in the basal compartment and secondary spermatogonia through spermatozoa are in the adlumenal compartment.
- Occasionally, cells that should reside in the adlumenal compartment and be separate from immunological agents are detected and the ensuing immunological response results in infertility.
Sertoli function stimulated by FSH
- Recall that the anterior pituitary releases FSH which binds to the FSH receptor on Sertoli cells.
- For related recall, LH is released by the anterior pituitary to bind on LH receptors of the Leydig cells found in the interstitium between the seminiferous tubules.
- FSH signaling on Sertoli cells causes phagocytic activity and production of several secretions.
- Secretions of the Sertoli cells:
- Activin and Inhibin: stimulate and inhibit the anterior pituitary cells to release FSH.
- Androgen binding protein (ABP): secreted into the lumen of the seminiferous tubule, binds up and concentrates testosterone.
- Tubular fluid: lubrication.
- Phagocytic activity of Sertoli cells is responsible for degrading residual bodies and malformed spermatozoa.
Leydig cells
- Leydig cells produce testosterone upon signaling from the anterior pituitary via LH.
- Recall that testosterone negatively feeds back on the hypothalamus.
- Note that Leydig cells do not secrete activin or inhibin.
- Leydig cells are found in clusters in the peritubular interstitium of the testis, between the seminiferous tubules.
- Recall that Leydig cells are often found near capillaries.
- Leydig cells are characterized by eiosinophilicism, lots of sER, mt with tubular cristae, and a lack of secretory vesicles.
- Regarding lots of sER, recall that steroids are generated in sER.
- Regarding a lack of secretory vesicles recall that steroids can pass directly through the membrane and therefore need not vesicular secretion.
- By transmission EM, one can also discern crystalline inclusions.
- Leydig cells are "transiently" active during development and then initiate full activity at puberty.
- Recall that Sertoli cells generate ABP (androgen binding protein) upon FSH signaling and Leydig cells produce testosterone upon LH signaling.
- It is by the parallel production of ABP and test (by Sertoli and Leydig cells) that testosterone is effectively delivered to the male reproductive tract.
Segments of the male reproductive tract
- The male reproductive tract begins at the seminiferous tubules and runs to the penile urethra.
- Seminiferous tubules -> tubuli recti (straight tubules) -> rete testis -> efferent ductules -> epididymal ducts -> ductus deferens (vas deferens) -> ejaculatory duct -> prostatic uretral -> membraneous urethra -> penile urethra.
- The seminiferous tubules reside in the lobules of the testis.
- The seminiferous tubule runs posteriorly and medially (toward the mediastinum) to the rete testis via the tubuli recti.
- The rete testis are a collecting system of the tubuli recti that dump into the efferent ductules.
- Efferent ductules (recall that the "e" of efferent means exiting) allow exit of the fluid to the epididymal duct (e-duct -> e-duct).
- The epididymal duct leads to the organ called the epididymis, which lies on the posterior, caudal aspect of the testis.
- Fluid travels from the epididymis superiorly via the ductus deferents.
- The tract becomes the ejaculatory duct once the seminal vesicle has joined; this makes sense if you think "now that we have seminal secretions (60% of the fluid) we're getting ready for ejaculation).
- The rest of the tract is the urethra and then named based on the structure through which the tract is laid: the prostate, then the membranous part of the penis, and then penis proper.
Where do basal cells and principal cells start?
- Straight tubule (tubuli recti)
- As the name implies, these are relatively straight tubules.
- Contributes to fluid
- Cuboidal to columnar
- Contains Sertoli cells
- Rete testis
- This is an elaborate network of channels.
- Contributues to fluid
- Has an irregular epithelial morphology: squamous, cuboidal, columnar.
- Has a fibrous stroma
- Efferent ductule
- The efferent duct connects the rete testis to the epidiymal duct and really does "exit" the testis proper and pass through connective tissue like the tunics.
- Like the rete testis, the efferent ductule has an irregular epithelial morphology and also has ciliated, pseudostratified cells.
- These ciliated pseudostratified cells are called principal cells and are found as clusters of columnar cells surrounded by short cells.
- These cells are the only ciliated cells of the male genital tract; it even makes a bit of sense that these are ciliated because they are passing through a sturdy connective tissue structure.
- The short cells that surround the columnar cells are absorptive cells.
- Ductus epididymis
- Leads to the organ called the epididymis.
- The ductus epididymis is characterized by an pseudostratifed columnar epithelium, stereocilia, and a prominent muscularis layer.
- The ductus epidiymis is pseudostratified epithelium (like the efferent ductule) and has stereocilia (which are different than cilia!).
- Note that stereocilia are not like cilia because they don't move and they are not like microvilli because they are not that small.
- The ductus epididymis is characterized by basal cells and principle cells.
- Basal cells are regenerative.
- Principal cells are secretory / absorptive.
- The ductus epididymis has a prominent muscularis layer that thickens distally and changes from just a circular layer to an inner circular and outer longitudinal layer.
- The functions of the ductus epididymis: absorb 90% of the tubular fluid, secrete factors that mature and capacitate sperm, and phagocytize cellular debris.
- Ductus deferens (vas deferens)
- The ductus deferens is characterized by a pseudostratified columnar epithelium, abundant sympathetic innervation, and three layers to the muscularis.
- The ductus deferens is pseudostratified (like the efferent ductule and ductus epididymis) and has stereocilia (like the ductus epididymis).
- The muscularis has now added an inner longitudinal muscle layer to the ductus epididymis's inner circular and outer longitudinal.
- The sympathetic innervation will be important for ejaculation which is facilitated by contraction of the ductus deferens's muscularis.
- Recall that Point and Shooting require Parasympathetic and Sympathetic innervation.
- The ductus deferens runs within the spermatic cord.
- Ejaculatory duct
- The ejaculatory duct begins where the vas deferens (ductus deferens) joins the seminal vesicle.
- Bilateral ejacualtory ducts (one from each testis) enter the prostate.
- Membranous urethra
- The membranous urethra is characterized by a pseudostratified epithelium that transitions to a stratified columnar epithelium.
- The membranous urethra crosses the muscular UG diaphragm.
- Penile urethra
- The penile urethra is characterized by a pseudostratified epithelium that transitions to a stratified columnar epithelium, perhaps continuing on to stratified squamous epithelium at the top.
- The penile urethra is also characterized by the presence of urethral glands which are clusters of mucus cells in the mucosa.
| Section | Epithelium | Appendages | Muscularis |
|---|---|---|---|
| Seminiferous tubule | stratified | none | none |
| Tubuli recti | cuboidal -> columnar | none | none |
| Rete testis | irregular: squamous, cuboidal, columnar | none | none |
| Efferent ductule | pseudostratified | cilia | none |
| Ductus epididymis | pseudostratified | sterocilia | circular -> circular (inner) + longitudinal |
| Ductus deferens | pseudostratified columnar | stereocilia | longit (inner) + circular + longit |
| Ejaculatory duct | pseudostratified columnar | ? | ? |
| Prostatic urethra | pseudostratified -> simple columnar | ||
| Membranous urethra | pseudostratified -> stratified columnar | none | longit (inner) + circular + longit |
| Penile urethra | pseudostratified -> stratified columnar -> stratified squamous | none | longit (inner) + circular + longit |
Accessory glands of the male reproductive tract
Seminal vesicles
- Seminal vesicles (paired bilaterally) are responsible for producing the largest portion of the semen.
- Seminal secretions are rich in fructose (for energy), prostaglandins, amino acids, and ascorbic acid.
- This generates a viscous mucoid secretion.
- The seminal vesicle has plenty of smooth muscle with which to force this secretion out into the ejaculatory duct.
- Seminal vesicle function and growth is mediated by testosterone.
- The vesicles are blind-ended pouches near the prostate and ductus deferens.
- Seminal vesicles are lined with pseudostratified columnar epithelium.
- This makes sense because they pump semen out into the GU tract at the ductus deferens and ejaculatory duct, both of which are pseudostratified.
- The seminal vesicles have mucosal arches which are highly folded, convoluted walls.
Prostate
- The prostate as an organ surrounds the base of the bladder.
- The prostate generates a secretion that becomes part of the semen and serves to condition the environment of the famale GU tract.
- The fribromuscular stroma surrounding the prostate is important for proper discharge of prostatic secretions during ejaculation.
- The prostatic secretion is a water, acidic mix of factors like zinc and fibrinolysin.
- Zinc inhibits macrophage activity.
- Fibrinolysin inhibits clot formation in the uterus.
- The prostate as a gland has alveoli connected via tubules; the tubules converge to generate a group of ducts that dump into the urethral crest.
- The urethral crest receives the ejaculatory ducts and becomes the prostatic urethra.
- The epithelium within the gland is irregular: pseudostratified to simple columnar.
- Corpora amylacea is a concentration of glycoprotein-rich secretion in the lumen of the gland that stains eosinophilic (because of the "protein-rich" part).
- The prostate has three concentric-like zones: central zone, transitional zone, and peripheral zone.
- The central zone of the prostate is the most medial and contains primarily periurethral mucosal glands.
- The central zone often stains the lightest of the zones.
- The transitional zone of the prostate is the middle zone and contains periurethral submucosal glands.
- Note that the central and transitional zones are most commonly associated with benign prostatic hypertrophy (BPH).
- The peripheral zone of the prostate is the outer zone, contains the main glands, is called the prostate proper, and is commonly involved with malignancies.
- The peripheral zone is often involved with prostate cancer.
Bulbourethral glands
- The bulbourethral glands produce a secretion for lubricating the male GU tract for ejaculation.
- The secretion of the bulburethral gland is clear and viscous.
Ejaculation sequence
- Ejaculation has a particular series of events regarding all these secretions:
- Bulbourethral glands discharge to lubricate.
- Prostate releases contents (via contraction of the fibromuscular stroma).
- Recall that the prostate's secretions serve to condition the female GU tract.
- The ductus deferens receives sympathetic stimulation to contract its muscularis (1->2 layers), thus pushing spermatozoa into the urethra.
- Seminal vesicles discharge their contents thus clearing the urethra by pushing semen distally.
- stopped here on 04/06/11.
Lab
- In the seminiferous tubules:
- Spermatogonia are right on the BM.
- Primary spermatocytes are light staining with dense chromosomes just adlumenal to the spermatogonia.
- We won't have to ID secondary spermatocytes.
- Spermatids are round cells with dense nucleus.
- Spermatozoa are elongated cells embedded in Sertoli cells.
- Sertoli cells have abundant cytoplasm.
- ID straight tubules by the presence of Sertoli cells.
- Ductus epididymis has very tall cells with cilia.
- Ductus deferens ID by stereocilia and three layers of muscle.
- Seminal gland: mucosal arches
- Prostate is ID'd by corpora amylacea (a concentration of glycoprotein-rich secretion in the lumen of the gland that stains eosinophilic).
- started here on 04/06/11.
Skin
- The skin is the heaviest, largest organ of the boyd.
- Skin is involved in protection, temperature regulation, hydration, excretion, and production of hormones, cytokines, and growth hormones.
Describe the basic histological structure of the skin
- There are 2 + 1 layers to the skin: the epidermis, the dermis, and the hypodermis.
- The epidermis originates from the ectoderm and is composed of epithelial cells.
- The dermis originates from the mesoderm.
- The hypodermis is not considered part of the skin proper and contains adipose tissue.


Identify the cell layers that constitute the epidermis
- The epidermis contains 4 layers in most locations and 5 layers on the hands and feet.
- The epidermis of the hands and feet is called thick skin and is hairless and contains a fifth layer.
- Also, the granulosa and corenum layers are thicker in "thick skin".
- From deep to superficial, the 5 layers of thick skin are: basale, spinosum, granulosum, lucidum, and corneum (BSGLC).
- The epidermis is not vascularized so the cells must rely on diffusion of nutrients from deep to superficial.
- Therefore, it makes sense that deeper cells are more alive and the shallower cells are more dead-like.
- Cellular progression from deep to superficial is also why it makes sense to talk about the layers from deep to superficial.


Stratum basale
- The stratum basale is also called the stratum germinativum.
- This base layer of epidermis is made of a single layer of mitotically active columnar or cuboidal cells.
- Recall that many adult stem cells live on basement membranes.
- The cells of the basal layer are connected to the basement membrane via hemidesmosomes.
/Integument/Thick%20Skin%20-%20Tutorial/F%20-%20Thick%20Skin%2040X-3-Epidermal%20Layers.jpg)
Stratum spinosa
- The spinosa appears as though there are a series of "spears" aligned within.
- This spinous look results from several layers of of cells connected by desmosomes.



Stratum granulosmum
- The stratum granulosum contains cells with granules which usually implies they have some specific role--and they do.
- The granule-containing cells of the granulosum contain filaggrin, and intermediat filaments that help to form the tonofibrils along with keratin.
- Note that these granules are called keratohyline granules.
- Kertohyline granules have no membrane.
- The cells of the granulosum are flattened polygonal cells--found in 3 to 5 layers.
- Epidermal cells of the granulosum are generally basophilic.
- Note that the granulosum is thickened in thick skin.
- There is a second type of granule (called lamellar granules) that contain lipids that are excreted into the connective tissue to provide a barrier to water-loss.
- Just like "lamellae" in chloroblasts, lamellar granules have a sort of stacked-bags look
- Labellar graunules cannot be seen in light microscopy.

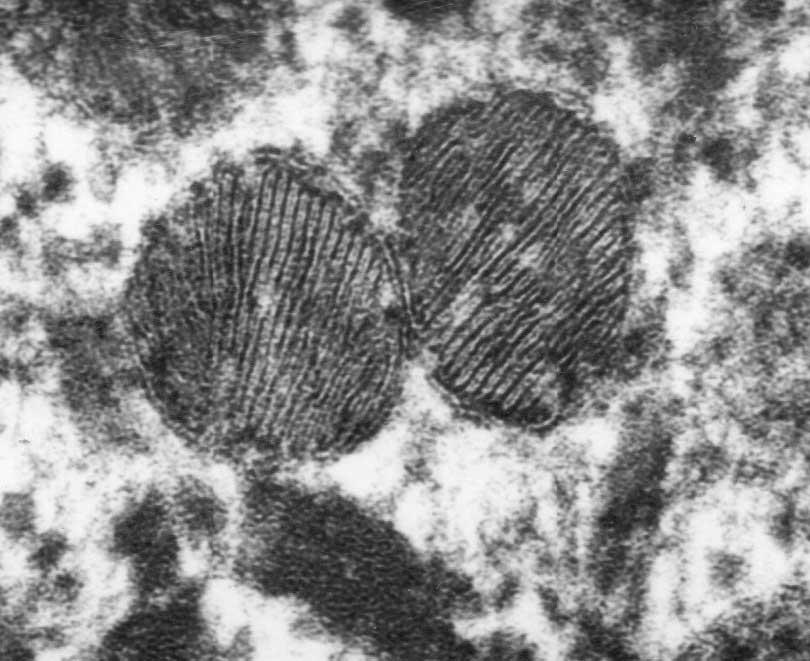


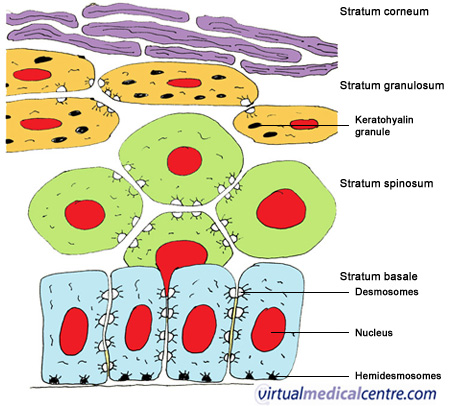
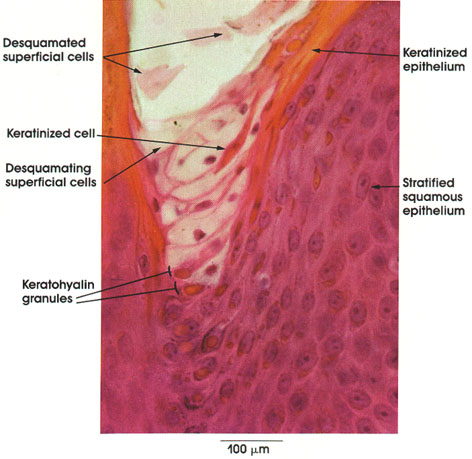
Stratum lucidum
- The stratum lucidum is only present in thick skin.
- The stratum lucidum contains only a few layers of very flattened eosinophilic epidermal cells.
- The lucidum (ironically) stains darkly.
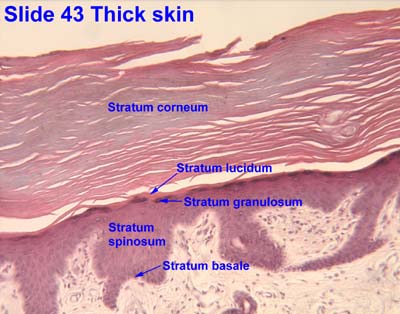
Stratum corneum
- The stratum corneum contains 15-20 layers of very thin, highly keratinized cells.
- These cells are called squames.
- Note that corneum means horny; think of a rhinocerus's horn--it is keratinized skin.
Dermo-epidermal junction
- Recall that the stratum basal is the deepest layer of the epidermis and that the dermis is deep to the epidermis.
- Recall, too, that the epidermal cells of the basal layer are connected to the basement membrane via hemidesmosomes.
- Recall that the dermis contains lots of connective tissue.
- The dermis contains lots of type 4 collagen.
- The superficial aspect of the dermis that is attached to the basement membrane of the epidermis is the lamina densa.
- The lamina densa of the dermis and the basement membrane of the epidermis are connected via anchoring filaments and anchoring fibrils.
- Note that anchoring filaments are composed of type 7 collagen.

Describe the cellular components of the epidermis and their functions
- There are four major cells of the epidermis: keratinocytes, melanocytes, langerhan cells, and merkel cells.
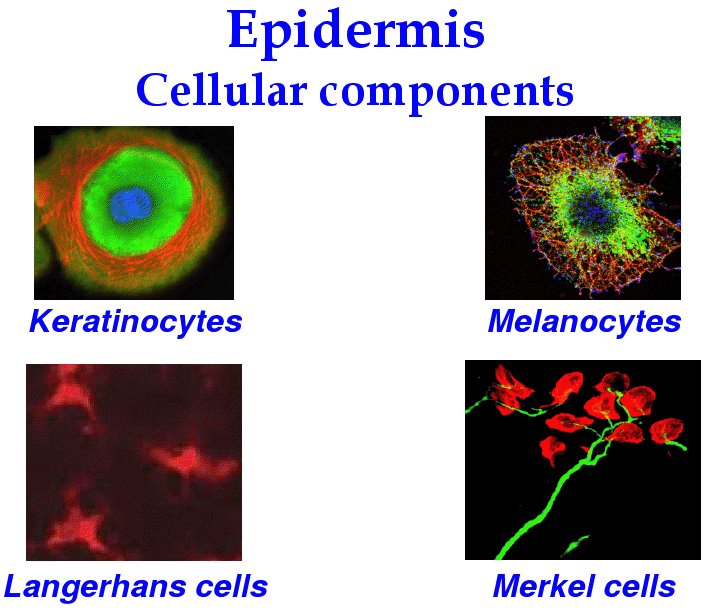
Keratinocytes
- Keratinocytes are the most numerous cell of the epidermis and primarily serve a structural role.
- Keratinocytes produce the protein keratin.
- Keratinocteys also help produce the water barrier with their tight junctions.
- Keratinocytes are found in all layers of the epidermis and take many shapes depending on their location.

Melanocytes
- Melanocytes reside in and near the stratum basale and have dendritic projections that weave through neighboring keratinocytes.
- Melanocytes are derived from neural crest cells and function to generate the pigment melanin which protects cells from UV damage.
- Melanocytes generate melanin and pass it to neighboring cells via cytocrine secretion upon MSH signaling.
- Note that melanin is produced from tyrosine via tyrosinase in a specialized intracellular organelle called a melanosome.
- Melanosomes are said to "mature" as they are produced; they turn from a light, circular shape to a dense cucumber shape.
- Eumalanosomes (black) and phaeomelanosomes (red / brown) give color to bird and dinosaur feathers.
- Albino organisms often lack tyrosinase and thus cannot generate melanin.
- Note that melanin is produced from tyrosine via tyrosinase in a specialized intracellular organelle called a melanosome.






Langerhans cells
- Langerhans cells are derived from bone marrow and thus it makes sense that they serve an immunological role.
- Langerhans cells bind, process, and present antigens to T cells with their star shaped cell bodies.
- Langerhans cells reside primarily in the spinosum layer (think "spines and chinese are for killing bad guys!).


Merkel cells
- Merkel cells (like melanocytes) are found primarily in the basal layer, which makes sense because they are a sensation cell that needs to be near a nerve ending.
- Note that Merkel cells are found primarily in thick skin where touch needs to be highly sensitive.
- Merkel cells, along with the expanded terminal bulb of afferent, myelinated nerves, form the Merkel's corpuscle which detects touch as a mechanoreceptor.
- The Merkel cells contain dense-cored neurotransmitter granules.

Describe the structural organization of the dermis
- Recall that the dermis is a connective tissue layer (collagen 4) that connects the epidermis to the hypodermis.
- The dermis has two layers (superficial to deep): papillary layers and reticular layer.
Papillary layer of the dermis
- The papillary layer is a delicate layer of connective tissue called papillary because of the papilla it forms as it protrudes up into the epidermis.
- Connective tissue of the papillary layer is composed of type 1 collagen, type 2 collagen, and elastic fibers.
- Recall that type 7 collagen binds the dermis to the basal lamina (the basement membrane) that separates the epidermis and dermis.
- It is the papilla between the epidermis and papillary layer of the dermis that generate fingerprints (dermatoglyphics).
- When you think of fingerprints think of Mark Twain and Juan Vucetich.
- The papillary layer contains nerves and vessels but they do not penetrate the epidermis.
Reticular layer of the dermis
- The reticular layer of the dermis is a sturdier connective tissue than the papillary layer and is less cellular.
- The reticular layer is composed of type 1 collagen and regularly oriented elastic fibers (called Langer's lines).
- The reticular layer is always thicker than the papillary layer; though the thickness of the reticular layer can vary depending on the location.





Identify other structures in the skin
Vessels
- The vascular layout of the skin serves two functions: to provide nutrient and waste exchange and to regulate body temperature.
- There are two plexuses of arteries: between the two layers of the dermis (papillary and reticular) and between the dermis and hypodermis.
- There are three plexuses of veins: between the two layers of the dermis (papillary and reticular) and between the dermis and hypodermis (just like the arteries) and a third plexus in the middle of the dermis.
- Recall that the reticular layer is thicker than the papillary layer so even though there is a vascular plexus between the two, there is also a veinous plexus deeper in the reticular layer.
- Arteriovenous anastomoeses serve as the shunts that can be opened or closed for temperature reasons--cooling or conserving, respectively.
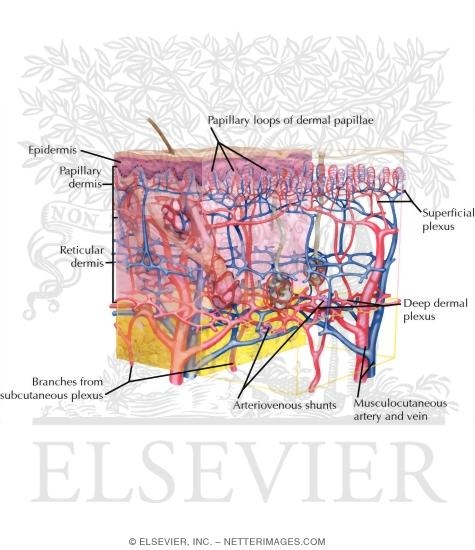

Sensory receptors
- There are four types of sensory receptors in the integumentary system (skin): free nerve endings, pacinian corpuscles, meissner's corpuscles, and ruffini's corpuscles.
Free nerve endings
- Free nerve endings are found in the stratum granulosum and detect fine touch, heat, and cold.
- Free nerve endings are not surrounded by connective tissue or schwann cells.


Pacinian corpuscles
- Pacinian corpuscles are nerve endings surrounded by an oval encapsulation of connective tissue in the deeper dermis and hypodermis.
- Pacinian corpuscles detect vibration.
- Pacinian corpuscles are myelinated.
- Think maraca shaped and all that vibration!






Meissner's corpuscles
- Meissner's corpuscles are found in the papillary layer of the dermis and are sensitive to low frequency stimuli.
- Doesn't "meissner" sound like an old miser with a low, grumpy voice?
- Meissner's corpuscles are shaped like tapered mitochondria and are oriented perpendicular to the skin.





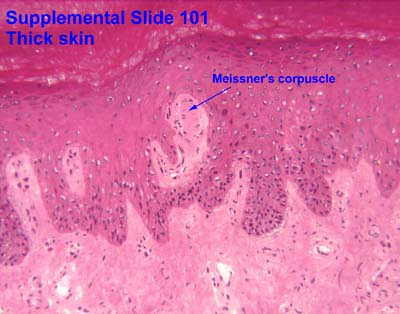


Ruffini's corpuscles
- Ruffini's corpuscles are simple mechanoreceptors and have an "elongated fusiform shape".

Sensory receptor images



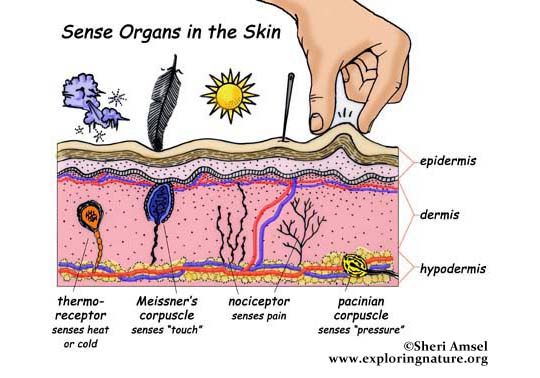
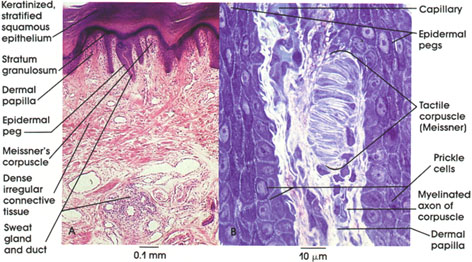


Hair follicles
- The hair follicle is responsible for production of hair is a structure composed of epidermal and dermal structures and cell types.
- Hair follicles originate from the invagination of epithelial cells downward to meet the papillary extension of dermal cells.
- At the hair follicle a specialized layer called the glassy membrane (a type of basement membrane that is thickened and keratinized) separates the epidermis and the dermis.
- Recall that a basement membrane separates the epidermis and dermis elsewhere, also, but that it is not keratinized.
- Note that the glassy membrane is acellular, like all basement membrane.
- During hair growth, the follicle has a bulbous end at the deepest part.
- Vasculature that arises from the dermal layer up through the dermal papilla services the bulb of the hair follicle.
- Surrounding the bulb (and originating from the epithelial layer) are melanoctyes which generate the melanin that dictates hair color.
So, why is pubic hair always dark, even if head hair is light? Do these melanocytes have more MSH receptors or some intracellular difference that generates a darker version of melanin?
- There are two sections to the root sheath: inner and outer.
- The inner root sheath runs from the bulb to the approximately the sabaceous gland.
- The outer root sheath runs from the sabaceous gland to the surface of the skin.
- Hair has three layers (from outside in): cuticle, cortex, and medulla.
- The cuticle is the outer most and is comprised of squamous cells.
- The cortex contains cuboidal cells that differentiate into keratinized cells.
- The medulla contains large cells with vacuoles that are moderately keratinized.
- Sebaceous glands are often associated with hair follicles and produce sebum as their secretion.
- Sebum is released into the infundibulum which is a pilosebaceous canal that surrounds the base of the growing hair.






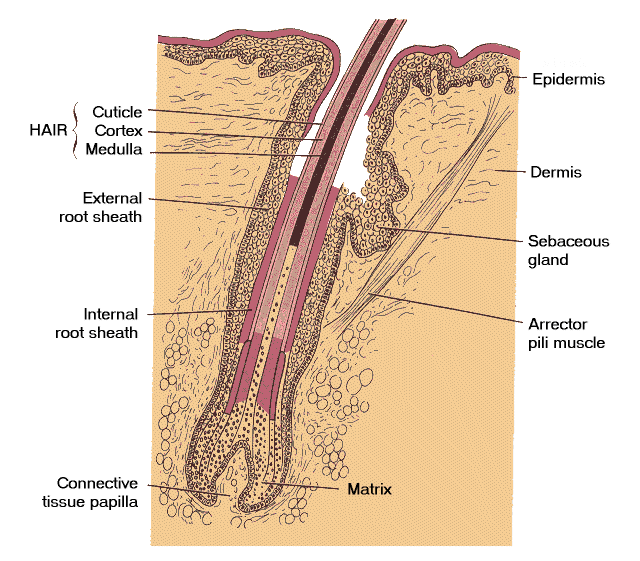


Phases of hair growth
- There are three phases of hair growth: anagen, catagen, and telogen.
- Anagen is active growth.
- Catagen is apoptosis-driven involution
- Telogen is the "resting phase" in which there is no more apoptosis but a new hair has yet to begin growing.

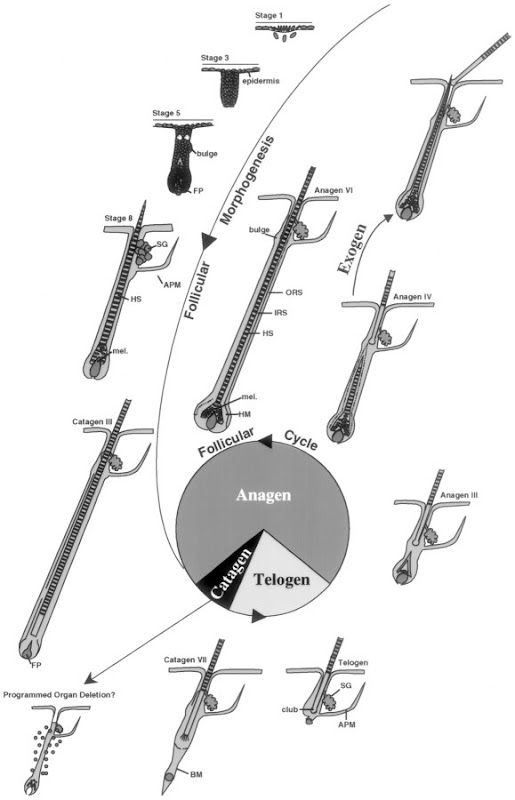
Nails
- The nail (the nail plate) is a structure of specialized stratum corneum that has hard keratin that does not desquamate like the skin corneum layer.
- The nail plate sit in the nail bed which is formed by the stratum basale and spinosum.
- Recall that the layers of the skin are normally BSGLC and here we have corneum sitting on spinosum and basal--the granulosum is missing.
- The nail bed is surrounded by the nail matrix which has a variety of cells: dividing cells that will cornify to become nail plate, melanocytes, merkel cells, langerhans cells, and stem cells.
- It makes sense that there are sensory cells in the nail matrix because sometimes you hit your nail plate just right and it hurts so bad!
- The nail root is the proximal end of the nail plate to which cornified cells are added to generate nail growth.
- The hyponichium is the point where the nail plate becomes free from the epidermis / nail bed.
- The epinichium is the part of the epidermis that covers the proximal end of the nail plate.



Glands
- There are two types of glands in the integument: sebaceous glands and sweat glands.
- Sebaceous glands use an holocrine method of secretion; that is, they produce more and more of their secretion and then rupture their cell membrane and spill the contents of the lumen.
- Sweat glands come in two forms: merocrine (eccrine) and apocrine.
- Merocrine (eccrine) secretion is the dumping of vesiclular content.
- Apocrine is the release of part of the cell by division of the cell membrane.

Sebaceous glands
- Sebaceous glands use holocrine secretion: the release of the contents of the lumen.
- Recall that sebaceous glands are often associated with hair follicles.






Sweat glands
- Sweat glands come in two forms: merocrine (eccrine) and apocrine.
| Attribute | Merocrine | Apocrine |
|---|---|---|
| Secretion method | Merocrine | Apocrine and merocrine |
| Distribution | Widely distributed | Axillary and perineal regions only |
| Lumen size | Small lumen | Large lumen |
| Epithelial type | Stratified cuboidal | Simple cuboidal |
| Innervation | Cholinergic fibers (ach) | Adrenergic (cats) |
- Merocrine (eccrine):
- Recall that eccrine (merocrine) secretion is the dumping of vesiclular content.
- Merocrine sweat glands are widely distributed

- Apocrine:
- Recall that apocrine secretion is the release of part of the cell by division of the cell membrane.
- Apocrine sweat glands are found in the armpits (axilla) and genital (perineal) regions only.


- Visual differentiation:
- Lumen size: small = merocirne, large = apocrine
- Density: denser = merocrine, lighter = apocrine
- Surrounding cells: myoepithelial cells surround merocrine to help secrete, apocrine don't necessarily have surrounding cells.
Understand the mechanism of skin repair
- Repair of a wound to the epidermis / dermis requires proliferation at both layers and the contribution of clotting.
- Increased vascular flow at the site of wound contributes to formation of a clot known as a scab, made of fibrin, which generates an initial scaffold for epidermal and dermal cells to follow in remodeling the area.
- The dermis is responsible for cleaning up the damaged cartilage (via macrophages) and proliferating new fibroblasts to generate new connective tissue.
- The epidermis contributes basal cells (recall that they are mitotically active) near the wound to migration and proliferation at the wound to generate new epithelial layers.
- When the whole epidermis and dermis are damaged, cells from the base of the hair follicles and sabaceous glands can migrate and proliferate to regenerate the dermis and epidermis.
- Indeed, we can even generate iPSCs from these adult stem cells.

Describe the histological findings in common skin diseases
- We will look at the histology of three classes of integument ills: blistering, psoriasis, and skin cancer.
Blistering
- Blistering occurs because of intercellular connection loss or because of a loss of connection at the epidermis-dermis junction.
- Abnormalities at the epidermis-dermis junction are called bullous pemphigoid.
- Abnormalities of intercellular junctions are called pemphigus.
Psoraisis
- Psoriasis occurs when cells of the basal and spinosum layers demonstrate excessive proliferation and decreased cycle time which leads to increased thickness.
- One can identify psoriasis by the presence of nuclei in the stratum corneum; this finding is called parakeratosis.
- Recall that cells of the stratum corneum do not usually have nuclei.
- Recall that we called the tongue epithelium perakeratinized because it had significant keratinization, differential staining, and yet the nuclei remained present.



Skin cancer
- There are three major types of skin cancer, named for the origin of the tumor cells: basal cell carcinoma, squamous cell carcinoma, and malignant melanoma.

Basal cell carcinoma
- Note the excessive proliferation of the basal cells often drives them downward into the dermis.





Squamous cell carcinoma
- Note the aberrant growth in the stratum spinosum.


Malignant melanoma
- Note the excessive number of melanocytes.
- Mohs surgery first removes the visible melanoma and then uses microscopic examination of the tumor site and a precise map and excision technique to remove the roots of the cancer.



Griscelli syndrome
- Griscelli syndrome is also called "silver baby syndrome".
- Griscelli syndrome can result from a defective Rab27a protein which is part of the transport complex that moves melanosomes along microtubules for cyotocrine passage to other cells.
- Patients with silver baby syndrome have hypopigmentation and also immunodeficiency.
- stopped here on 04/06/11.
- started here on 04/11/11.
- NBME:
- 1/4 of the 100 questions are images.
- No questions on the eye or ear.
The eye
Optical anatomy
- Important anatomical features of the eyeball include:
- cornea with limbus (outer rim that holds stem cells)
- anterior chamber
- pupil
- iris
- posterior chamber
- ciliary body
- lens (with ciliary processes and zonules)
- vitreous body
- retina
- ora serrata
- fovea
- optical disc

Wall of the eye
- The wall of the eye is comprised of three layers: fibrous tunic, uvea, retina (where the retina is the inner-most layer).
- The fibrous tunic generates the cornea, the sclera, and provides continuity with the conjunctiva.
- The conjunctiva is a clear mucous membrane that lines the exterior of the sclera and cornea and is continuous along the poster aspect of the eyelid.
- The uvea is the vascular tunic (tunica vasculosa) that carries the vasculature of the eye.
- The uvea also makes the iris, ciliary bodies, and the choroid.
- The choroid is highly vascularized.
- The retina provides the sensory material of the eye.
- The retina includes the pigmented epithelium, the neural photoreceptor cells, and the neuronal integreative circuitry and supporting cells.
- Recall that photoreceptor cells come in rods and cones.
- Note that the pigmented epithelium is deep to the photoreceptors.

Eyeball function by component

Cornea
- The cornea is the most superficial layer of the eye and is exposed to the environment.
- Recall that the cornea arises from the fibrous tunic of the optical tissue.
- The cornea is transparent, avascular, and bends the incoming light to hit the lens.
- In fact the cornea bends the light (refracts) even more than the lens.
- Photorefractive keratectomy and LASIK (laser in situ keratomileusis) are two surgeries that reshape the deeper (non-epithelial) layers of the cornea to correct poor refraction that results in poor vision.
- The cornea has three cellular layers and two atypical basement membranes.
- The layers (superficial to deep): epidermis, Bowman's membrane, stroma, Descemet's membrane, and endothelium.
- With age, Descemet's membrane decreases in transparency and leads to decreased light transmission.

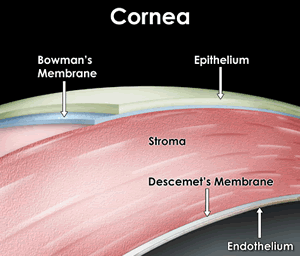


Epithelium of the cornea
- The epithelium of the cornea is made of stratified, squamous, non-keratinized epithelium.
- The epithelium layer is highly proliferative and has abundant pain fibers.
- The epithelial layer maintains a population of stem cells in the limbus area (the junction of the cornea with the sclera).
- Note that aqueous humor (from the anterior chamber) drains through the corneoscleral junction (the limbus).
- We call the corneoslceral junction (the limbus) a trabecular meshwork and the site of Schlemm's canal.



Stroma of the cornea
- The stroma is also called the substantia propria.
- There are 50-60 layers of cells making the stroma the most substr
- The stroma is series of layers of fibrocytes, proteoglycans, and ECM fibers with alternately oriented collagen fibers.
- Note that collagen of the stroma is of type 1 and 5 and are non-fibrillar.
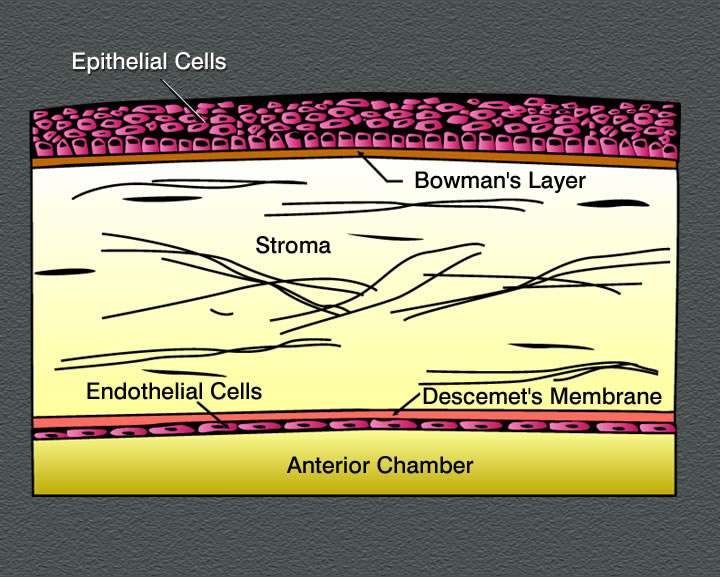
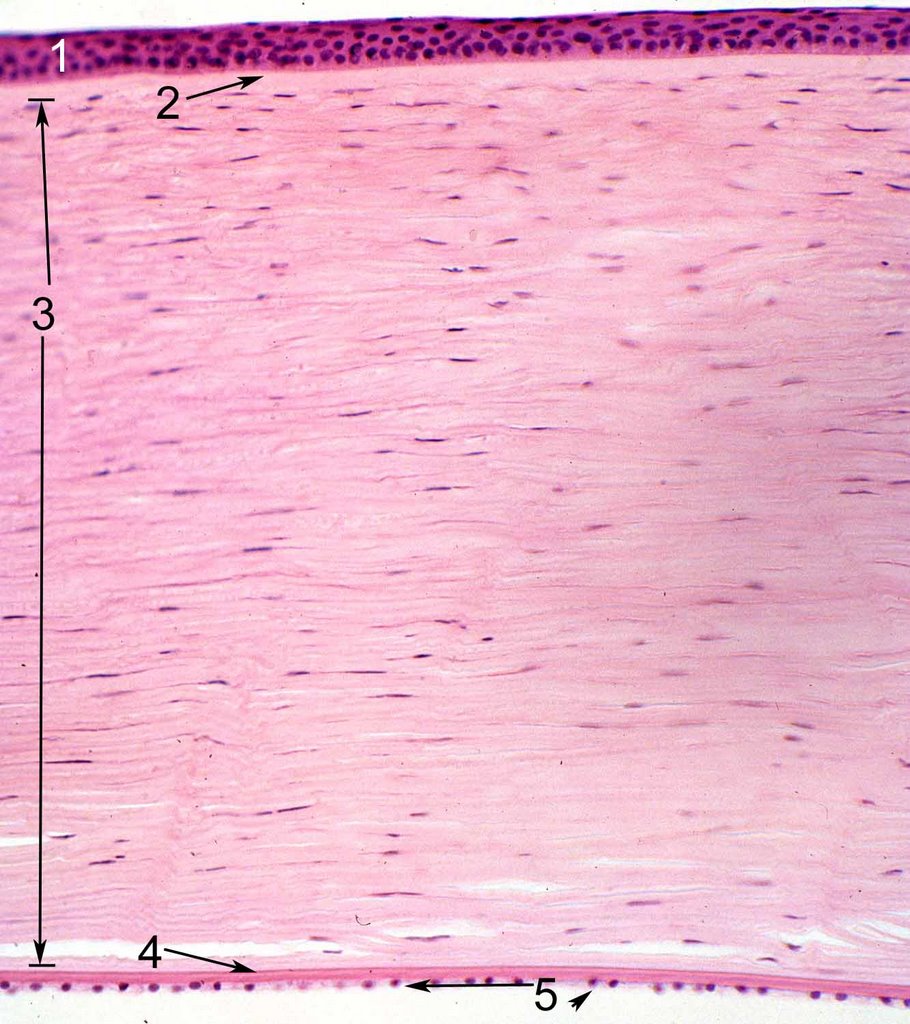

Endothelium of the cornea
- The endothelium of the cornea contains leaky intercellular junctions that allow exchange of fluid with the anterior chamber.
- Drainage by the endothelium keeps the stroma relatively dry.
- Note that fluid transport by the endothelium contributes to the transparency of the cornea.

Uvea
- Recall that the uvea is one of the three layers of the eye: the tunica fibrous, the uvea, and the retina.
- Recall that the uvea is also called the tunica vasculosa and carriers the vasculature of the eye.
- The uvea includes the iris, the ciliary bodies, and the choroid.
- Just as the cornea is the anterior portion of the fibrous tunic and is completed posteriorly by the sclera, the iris and ciliary body are the anterior aspect of the tunica vasculosa and are completed posteriorly by the choroid.

Choroid of the uvea
- Recall that the choroid is the posterio-lateral aspect of the uvea (tunica vasculosa).
- The choroid carries vasculature to the retina and the sclera.
- The choroid is highly pigmented and found between the sclera (part of the tunica fibrous) and the retina.
- The choroid has three layers: sclera vasculature, retinal vasculature, and Brunch's membrane.
- The retinal vasculature layer is also called the choriocapillary layer.
- Note that these layers are indistinguishable in the lab.
- The choriod layer is separated from the retina by the Bruch's membrane; that is, Brunch's membrane is the deepest aspect of the choroid part of the tunic vasculosa (uvea).
- Bruch's membrane is also sometimes called a glassy membrane.
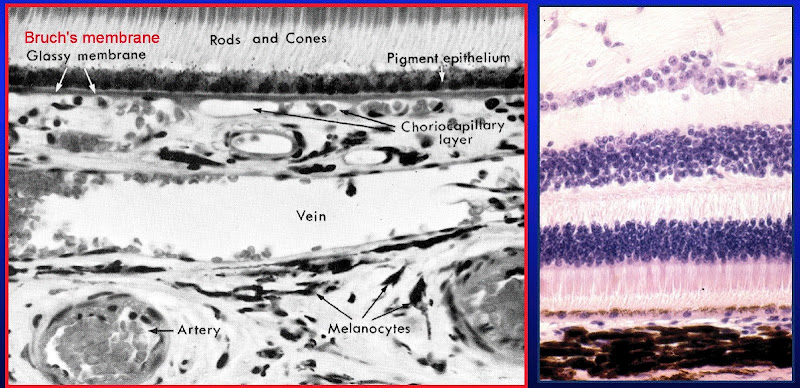

Ciliary body
- The ciliary body is a thickening of the vascular layer near the anterior aspect, radially from the lens.
- Ciliary bodies have two structures: ciliary zonules (ligaments) and ciliary processes.
- Ciliary zonules (suspensory ligaments):
- Ciliary ligaments project from the tunica vasculosa to the lens capsule and control the flattness / bulging of the lens in order to focus light (a process called accomodation.
- Upon contraction of the ciliary muscle, the many ciliary zonules are brought closer together and anterior which actually allows the lens to relax.
- Think of the ciliary muscle as a sphincter and the relationship with zonules and the lens makes sense.
- Far vision requires a flattened lens (think flat like a frisbee which you hope will go "far") so the ciliary muscle relaxes, the zonules tense, and the lens is pulled into a flatter shape.
- Near vision requires a bulged lens so the ciliary muscle contracts, the zonules relax, and the lens relaxes into a bulge.
- Recall too that near vision gives eye strain which is the excessive contraction of the ciliary muscles.
- Oxytalin fibers are used to attach the basement membrane of the non-pigmented epithelium of the ciliary body (part of the uvea) to the basement membrane (called the lens capsule) of the lens.

- Ciliary processes:
- Ciliary processes primarily function to generate aqueous solution.
- Aqueous solution provides nutrition to the lens and maintains intraocular pressure.
- Aqueous solution has little protein, some glucose, and similar ion concentration as plasma.
- Ciliary processes are an extension of the tunica vasculosa (the uvea) and are highly vascularized.
- There are two layers to the ciliary process tissue: deep layer (pigmented) and surface layer (secretory, non-pigmented).
- The pigmented layer (deep layer) of the ciliary process is continuous with the pigmented layer of the retina.
- The non-pigmented layer's (secretory, surface layer) apical surface faces the pigmented layer and the basolateral surface has lots of folds and borders the posterior chamber.
- We call the space between the pigmented and non-pigmented cells the ciliary channel and consider it a potential space.
- Blood-aqueous barrier: occluding junctions at the apex of the ciliary process's surface epithelium keeps blood and aqueous solution from mixing.
- Note that glaucoma results from blocked drainage of aqueous fluid and the resulting elevation of intraoccular pressure.
- Ciliary processes primarily function to generate aqueous solution.
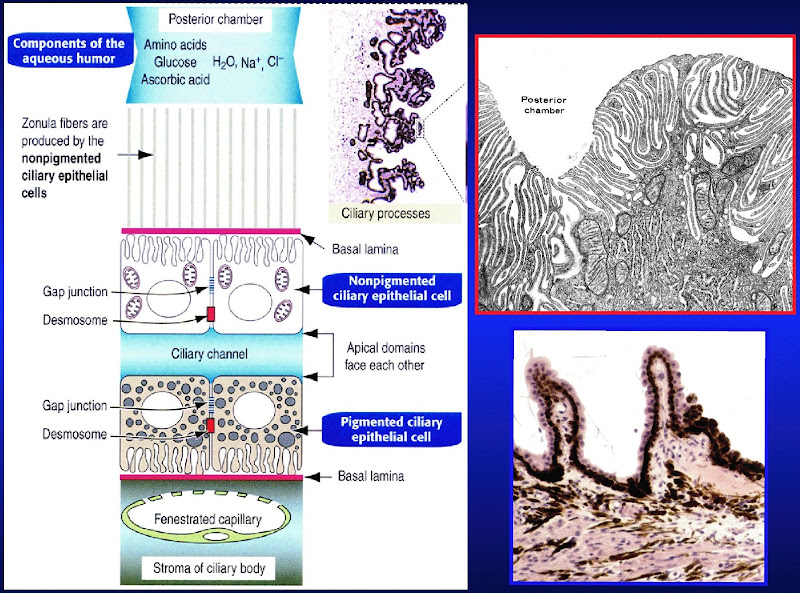

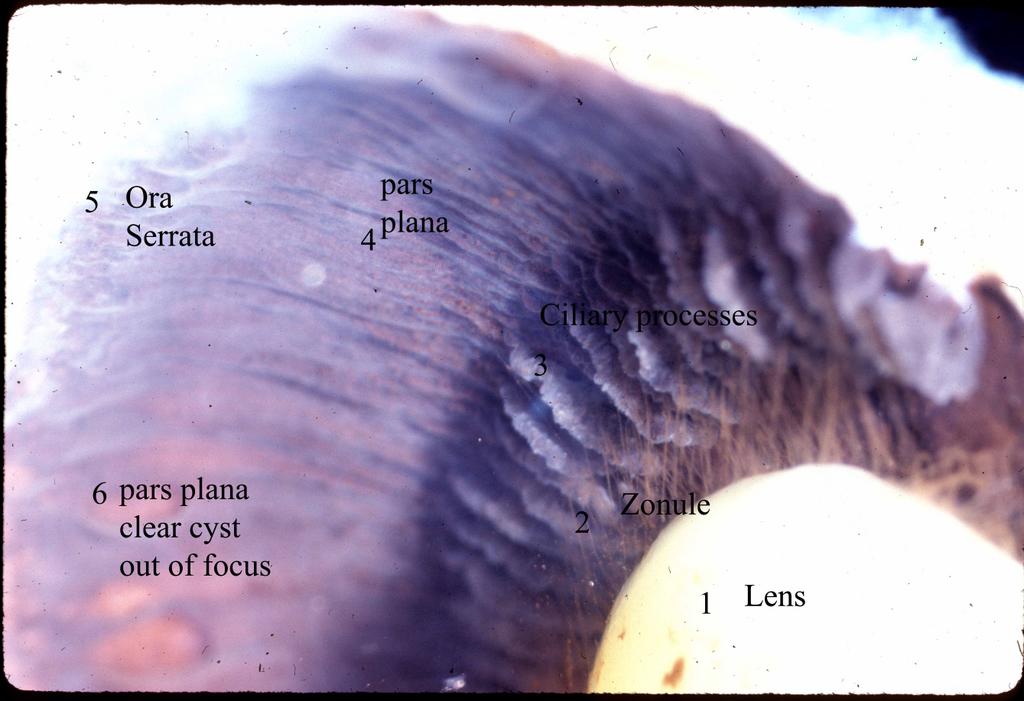

.jpg)
Iris
- The iris the most anterior aspect of the uvea (the vascular layer).
- Recall that the uvea is the middle layer of the three layers of the eyeball: tunica fibrous, tunica vasulosa (uvea), and the retina.
- The iris divides the eyeball into two chambers: the anterior and posterior chambers.
- The iris's job is to regulate the amount of light entering the eye ball.
- The posterior aspect of the iris has epithelial and myoepithelial layers that provide the pigment of eye color and the movement of the iris, respectively.
- Note that the posterior epithelium is highly pigmented but does not determine eye color.
- Note that the posterior epithelium is one and the same as the myoepithelium: it has muscle fibers in it and is responsible for dilating the pupil.
- The iris is primarily composed of stroma with an epithelial covering only on the posterior surface.
- The stroma is highly vascular (recall that the iris is part of the tunica vasculosa) and has fibroblasts, connective tissue, melanocytes, and myocytes.
- The malanocytes of the stroma determine eye color.
- The stroma is responsible for constricting the pupil.
- Note that the stroma contains the constrictor muscle of the pupil and the myoepithelium contains the dilator muscle of the pupil.
- Note that dilation is sympathetic and contraction is parasympathetic.
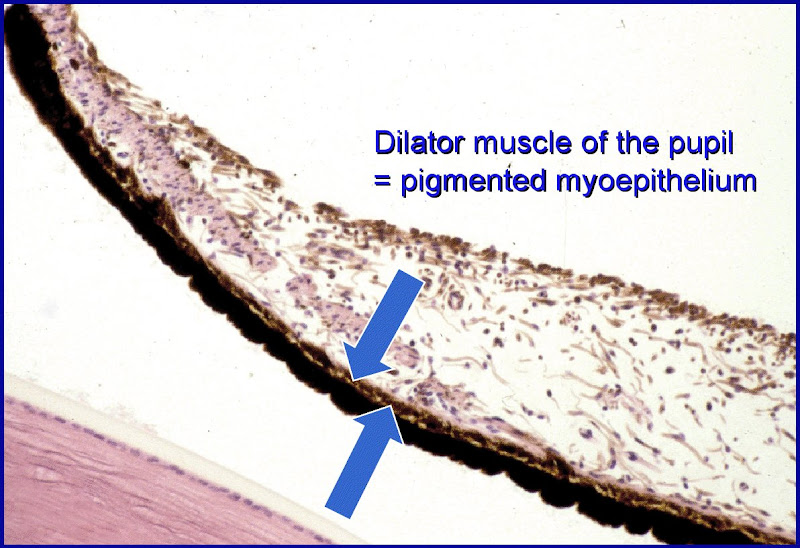
Eye color
- Eye color is determined by the quantity and distribution of pigment cells in the stroma and posterior epithelilum of the iris.
- Pink: complete lack of pigment; albinism (lack of tyrosinase or a defect in melanosome transport, RAf27a)
- Blue < grey / green < brown => less to more pigment.
- Heterochromia iridium is the presence of distinct regions of different color in the iris.
Lens
- The lens of the eye is biconcave, transparent, and avascular.
- The lens capsule is the basement membrane of the lens and sits on the outer surface.
- The lens has an epithelium but only on the anterior surface just as the iris only has an epithelium but only on the posterior surface.
- The epithelium on the anterior side of the lens is simple cuboidal.
- Note that the lens epithelium does not have occluding junctions.
- The stroma of the lens is composed of highly specialized cells (called lens fibers) that span the anterior-posterior axis and refract light well because of their high concentration of crystallin a cellular protein.
- The cells at the center of the lens contain the highest concentration of crystallin.
- These stromal cells are 7-10 mm in length.

- In the case of cataracts, lens fibers (the cells that span ant-post and have crystallin) turn opaque and refract light poorly.
- In the case of presbyopia, the lens loses elasticity (some loss is normal during aging) such that the pt cannot accomadate well and thus has poor near vision.
Retina
- The retina is the inner-most of the three layers of the eye (tunic fibrous, tunica vasculosa, and retina).
- The retina has two functional layers: the pigmented epithelium on the outside (next to Brunch's membrane from the tunic vasculosa = uvea) and the neural retina.
- Note that the neural retina and the pigmented epithelium are not well connected and thus retinal detachment often occurs between these two layers.
- The epithelium is essentially a support tissue for the neural retina.
- The unlike the tunica fibrous and (almost) the tunica vasculosa, the retina does not continue all the way anteriorly.
- The anterior apex of the retina is called the ora serrata.
Pigmented epithelium of the Retina
- The pigmented epithelium of the retina is simple cuboidal and is adjacent to the inner-most layer of the uvea (Bruch's membrane).
- Recall that Bruch's membrane is also sometimes called a glassy membrane.
- The pigmented epithelium serves to support the neural retina (which is deeper) and thus its apical membrane faces the neural retina:
- secretes nutrient-rich fluid onto the nueral retina
- Note that secretion is enabled via Na-K ATPase.
- produces melanin to absorb light and reduce scatter
- cleans up material shed by rods an cones (phagocytosis, discs)
- concentrates and estrifies vitamin A for easy reproduction of rhodopsin by rods
- secretes nutrient-rich fluid onto the nueral retina
Neural retina
- The neural retina is the inside (deep) layer of the retina's two layers (pigmented epithelium and neural retina).
- The cells that make up the neural retina include: photoreceptors (rods and cones), supporting cells, and integrative neurons.
- Rods and cones have their own anatomy related to their function (from closest to the field of view to closest to the brain): synaptic body, inner segment, outer segment.
- The synaptic body is in contact with bipolar cells of the retina.
- Note that the location of synapse from the photoreceptor to the bipolar cell is closer to the field of view than the location of photoreception.
- The inner segment:
- The outer segment contains the photosensitive apparatus, has highly modified cilium, and is in contact with the pigmented epithelium.
- Note that the outer segment is closes to the brain.
- The synaptic body is in contact with bipolar cells of the retina.


- Rods versus cones
- Rods are specialized for low light vision while cones are specialized for high light and color vision.
- "C" is for color.
- Rods use rhodopsin while cones have three distinct pigments for three colors: red, green, and blue.
- Rods are associated with the "peripheral" areas of the eye while the cones are found in higher density at the "center" region.
- Note that "peripheral" and "central" are quoted because we don't really mean the "center" we mean near the fovea centralis--the focal point of highest acuity.
- It makes sense that the cones are concentrated in the "central" area because they are high acquity photoreceptors and the center is where our focal point usually sits.
- The fovea is the cones-only area of the retina and the location of highest visual acquity.
- Rods are specialized for low light vision while cones are specialized for high light and color vision.
| Attribute | Rods | Cones |
|---|---|---|
| Function | Night vision (scotopic), low acquity | Day vision (photopic), color, high acquity |
| Photopigment | Rhodopsin | 3 pigments, 3 wavelength specificities |
| Distribution | "Peripheral" | "Central" |
| Population count | 120 million | 6 million |
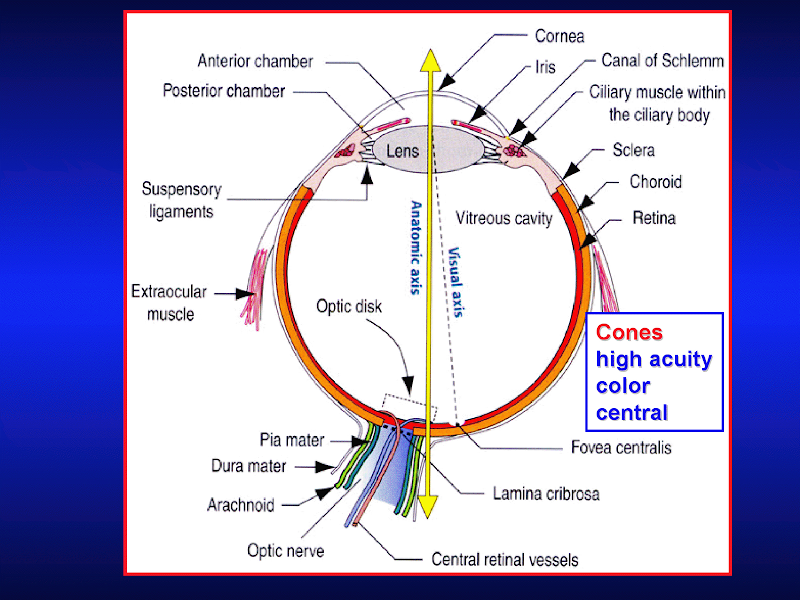


Lab
- Note that the Bowman's membrane in the cornea is large and thick and separates the stroma from the epithelium.
- stopped here on 04/11/11.





